Real Gases Deviation from ideal gas Law Gas










- Slides: 27

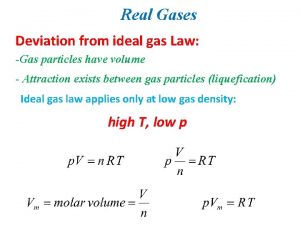
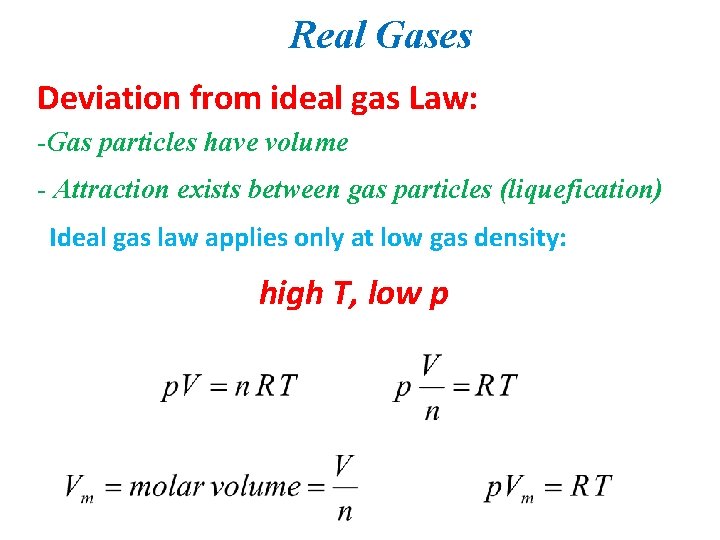
Real Gases Deviation from ideal gas Law: -Gas particles have volume - Attraction exists between gas particles (liquefication) Ideal gas law applies only at low gas density: high T, low p

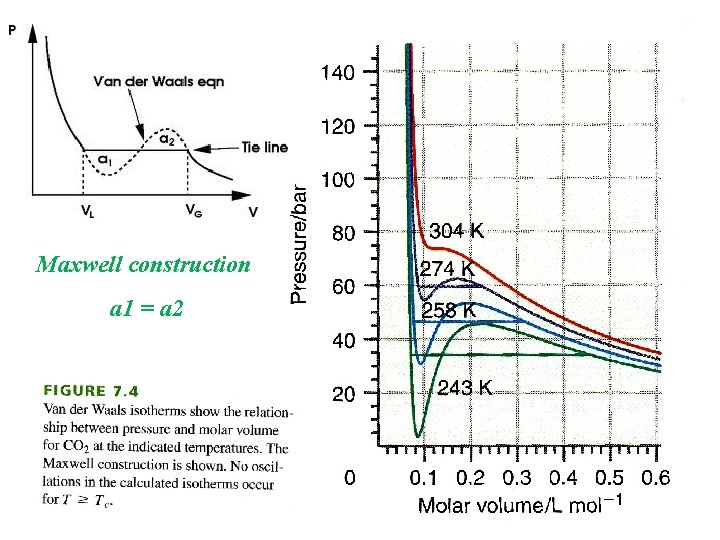
isotherm

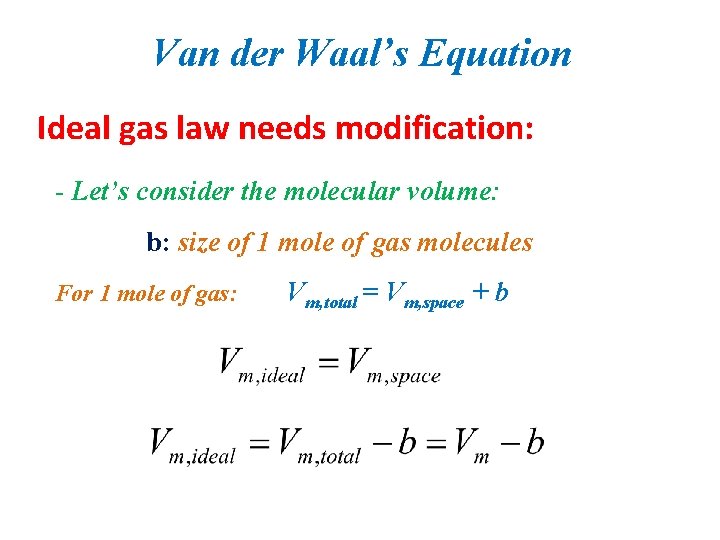
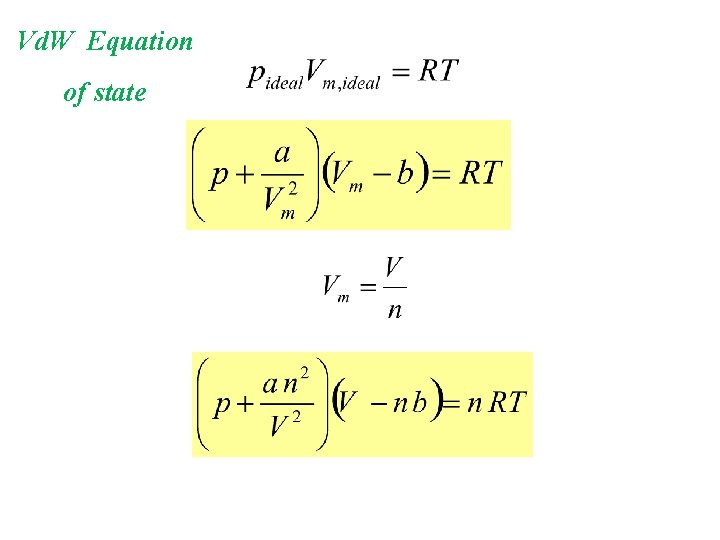
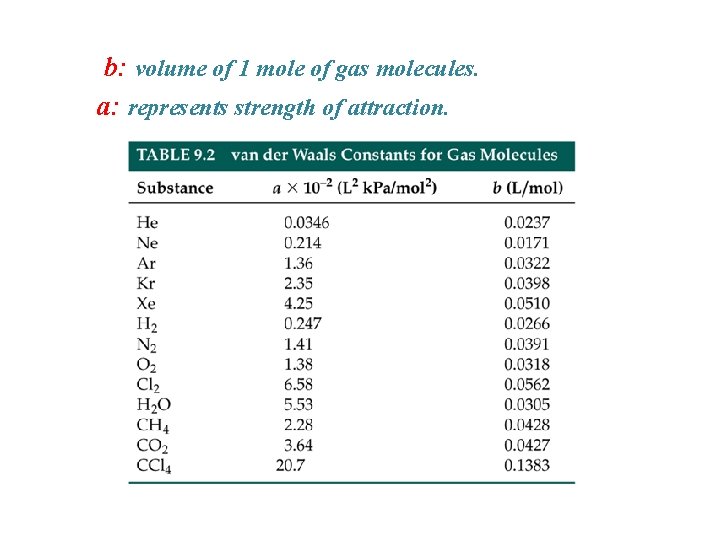
Van der Waal’s Equation Ideal gas law needs modification: - Let’s consider the molecular volume: b: size of 1 mole of gas molecules For 1 mole of gas: Vm, total = Vm, space + b

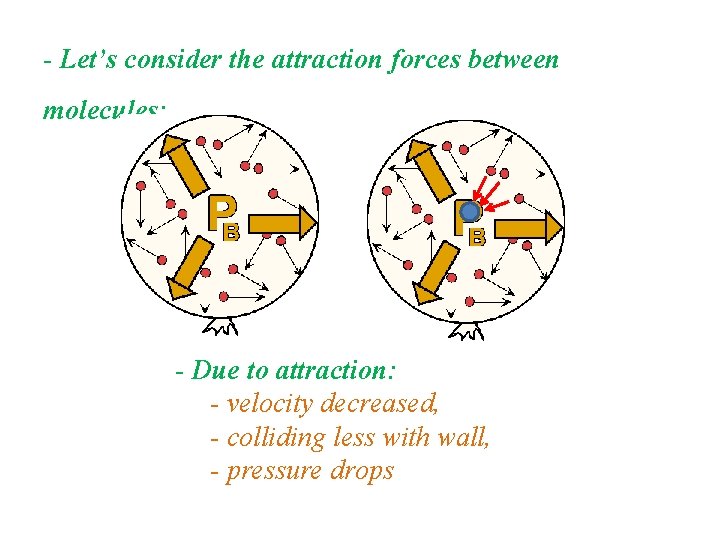
- Let’s consider the attraction forces between molecules: - Due to attraction: - velocity decreased, - colliding less with wall, - pressure drops

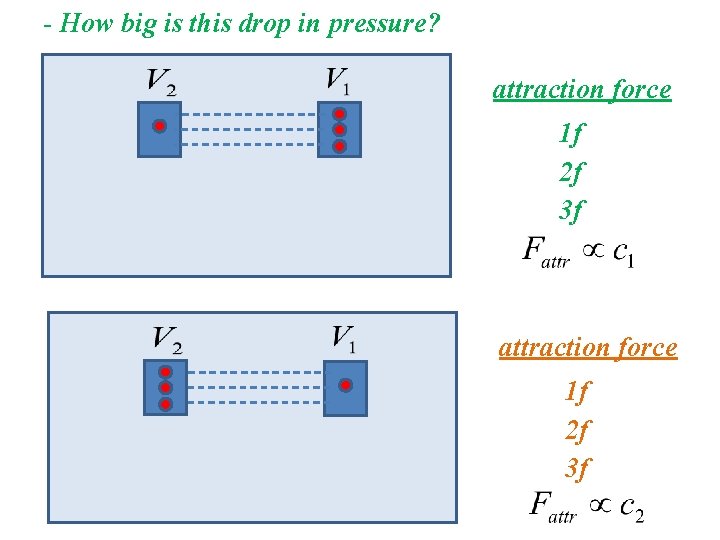
- How big is this drop in pressure? attraction force 1 f 2 f 3 f

- How big is this drop in pressure?

- How big is this drop in pressure? drop in pressure proportional to attraction force drop in pressure indirectly proportional to square of Vm

Vd. W Equation of state

b: volume of 1 mole of gas molecules. a: represents strength of attraction.


Maxwell construction a 1 = a 2

Many other equations of state Mostly empirical Virial Equation to correct, develop in a series (additional correction terms: B, C, …: second, third, … virial coefficients: exp. determined

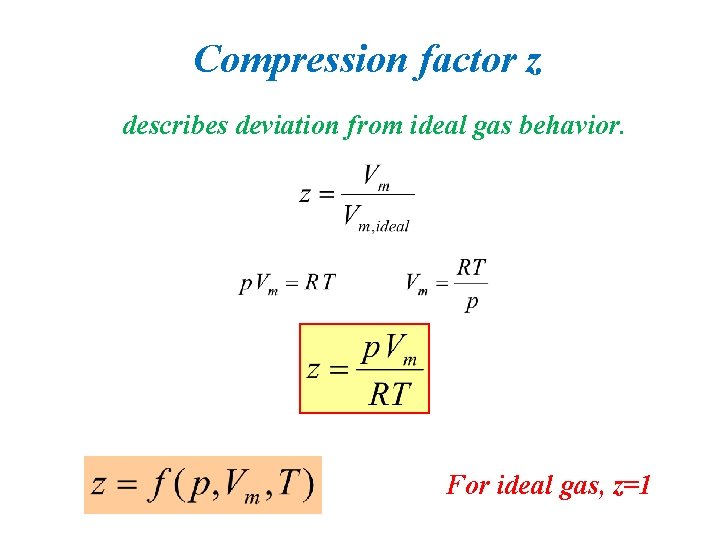
Compression factor z describes deviation from ideal gas behavior. For ideal gas, z=1

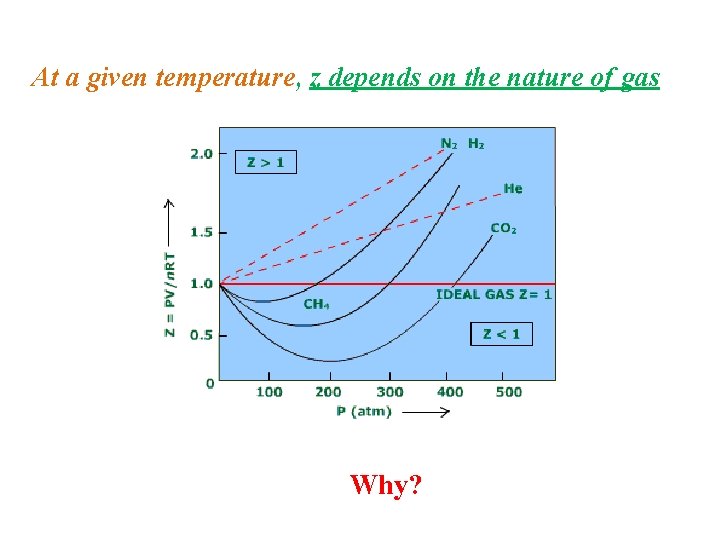
At a given temperature, z depends on the nature of gas Why?

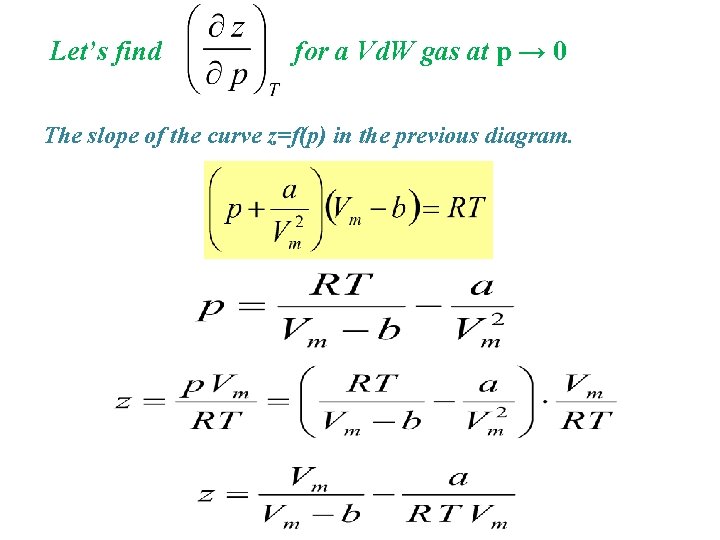
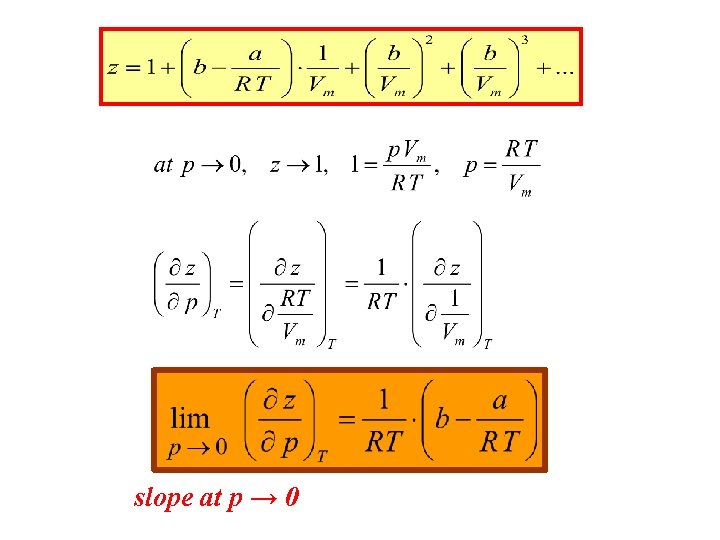
Let’s find for a Vd. W gas at p → 0 The slope of the curve z=f(p) in the previous diagram.


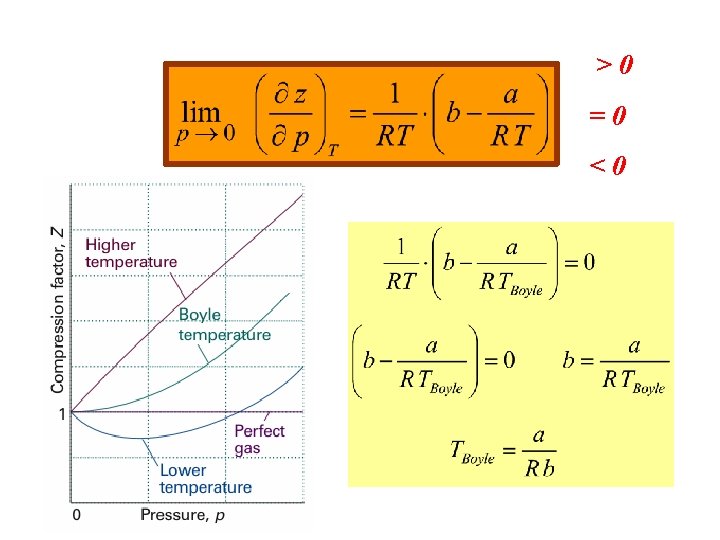
slope at p → 0

>0 =0 <0

Size effect > attraction Size effect = attraction Size effect < attraction b: volume of 1 mole of gas molecules. represents repulsive forces a: represents strength of attraction

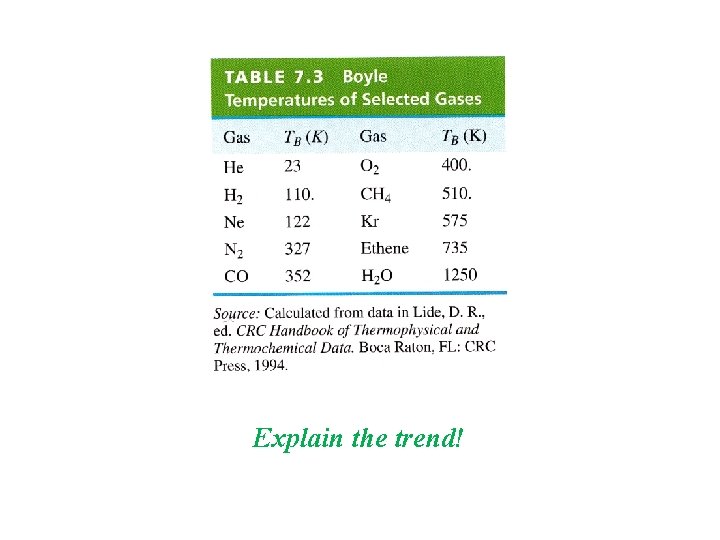
Explain the trend!

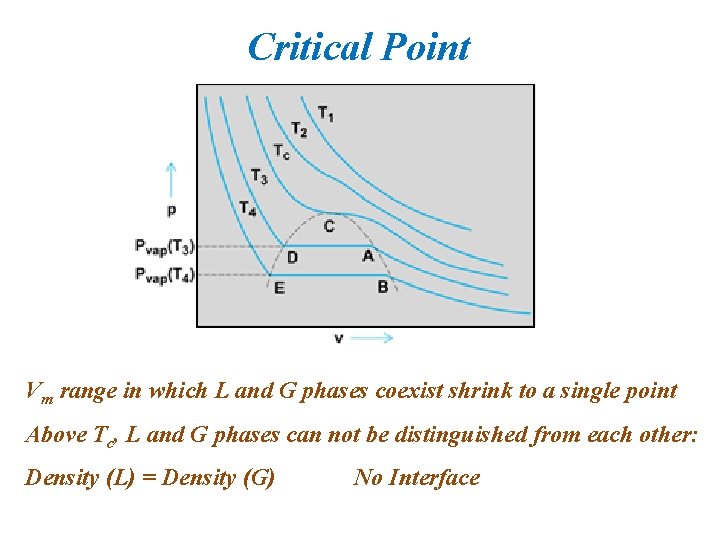
Critical Point Vm range in which L and G phases coexist shrink to a single point Above Tc, L and G phases can not be distinguished from each other: Density (L) = Density (G) No Interface


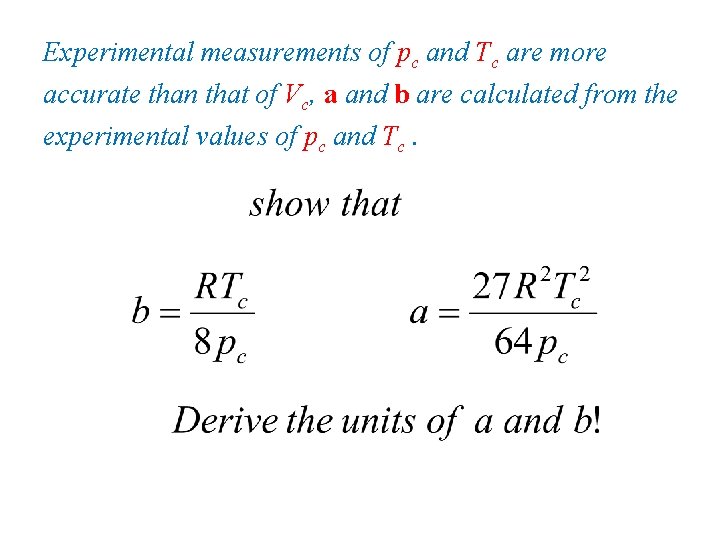
Experimental measurements of pc and Tc are more accurate than that of Vc, a and b are calculated from the experimental values of pc and Tc.

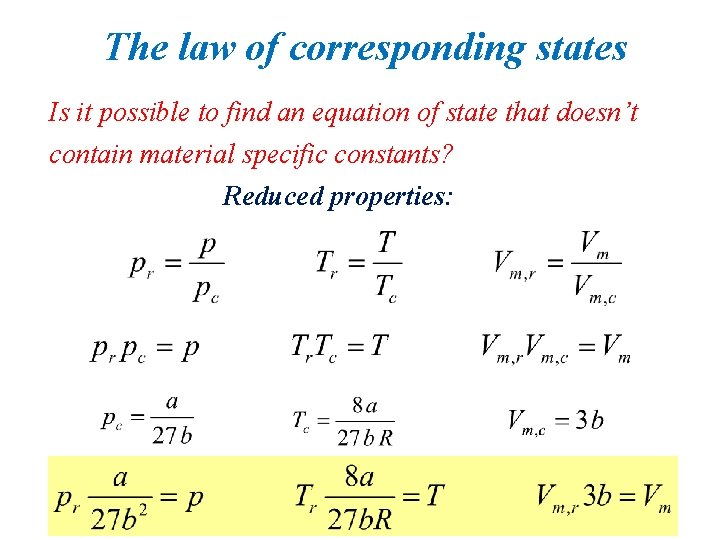
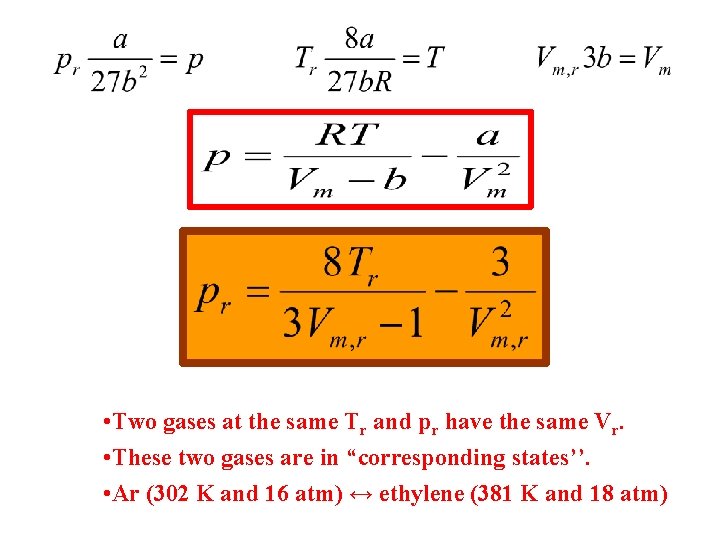
The law of corresponding states Is it possible to find an equation of state that doesn’t contain material specific constants? Reduced properties:

• Two gases at the same Tr and pr have the same Vr. • These two gases are in “corresponding states’’. • Ar (302 K and 16 atm) ↔ ethylene (381 K and 18 atm)


 Deviations from ideal gas law
Deviations from ideal gas law Gas law
Gas law Difference between ideal gas and real gas
Difference between ideal gas and real gas Derive ideal gas equation
Derive ideal gas equation An ideal gas is an imaginary gas
An ideal gas is an imaginary gas Sutherland's law
Sutherland's law Ideal gas law with mass
Ideal gas law with mass Gas laws formulas
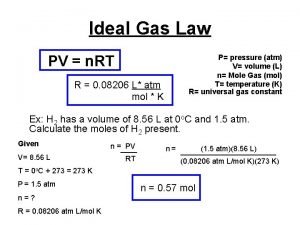
Gas laws formulas Ideal gas law
Ideal gas law Which equation agrees with the ideal gas law?
Which equation agrees with the ideal gas law? Ideal gas law examples
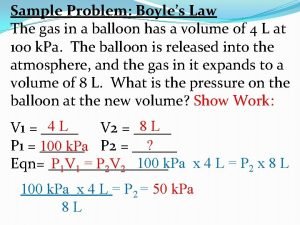
Ideal gas law examples Deviations from the ideal gas law
Deviations from the ideal gas law Pvn=rt
Pvn=rt Ideal gas law with density
Ideal gas law with density Conditions for ideal gas
Conditions for ideal gas Pv nrt units
Pv nrt units Volume of a gas formula
Volume of a gas formula Ideal gas law formula
Ideal gas law formula How to find density in ideal gas law
How to find density in ideal gas law Ideal gas law to find density
Ideal gas law to find density Pzmore
Pzmore A student collects oxygen gas by water displacement
A student collects oxygen gas by water displacement Gas laws
Gas laws Ideal gas law derivation
Ideal gas law derivation Ideal gases characteristics
Ideal gases characteristics Avogadro's law
Avogadro's law N in ideal gas law
N in ideal gas law Charles' law worksheet with answers
Charles' law worksheet with answers