What are Characteristics of a GAS Ideal Gases












- Slides: 33


What are Characteristics of a GAS?

Ideal Gases Real Gases have no mass. Gases have no volume. Gases have volume. Gases do not interact – elastic collisions. Assumptions Gases exert forces on each other. Real World

What does PRESSURE mean? • In Life: Pressure = a stoichiometry quiz every day • In Science: Pressure = force per unit area P= F A

How else can we measure Pressure?

Standard Temperature & Pressure 1 atmosphere 273 K (atm)

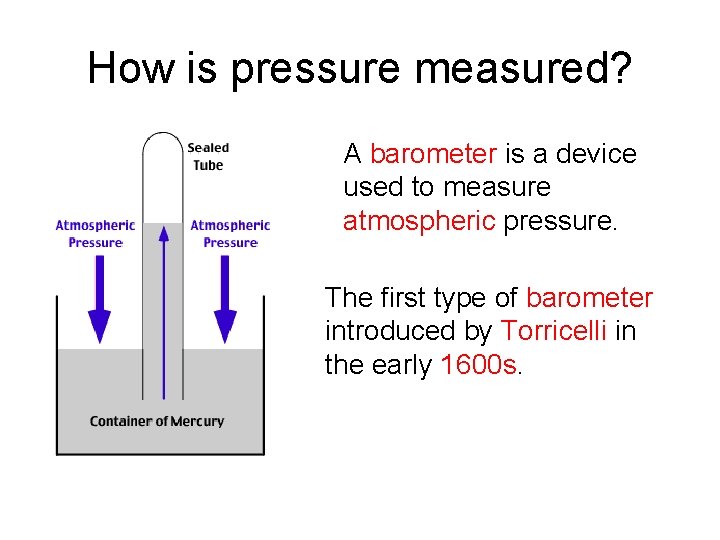
How is pressure measured? A barometer is a device used to measure atmospheric pressure. The first type of barometer introduced by Torricelli in the early 1600 s.

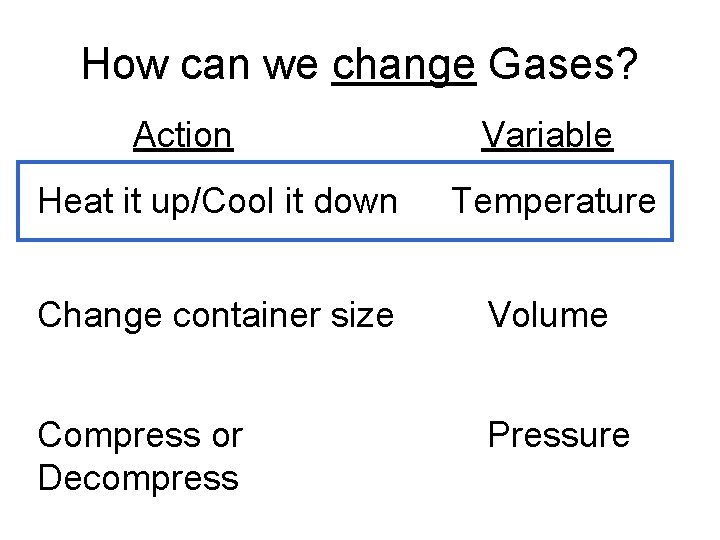
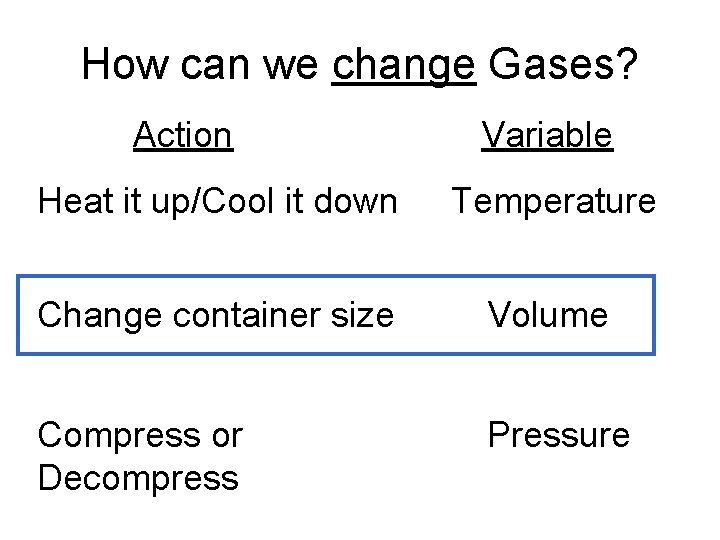
How can we change Gases? Action Variable Heat it up/Cool it down Temperature Change container size Volume Compress or Decompress Pressure

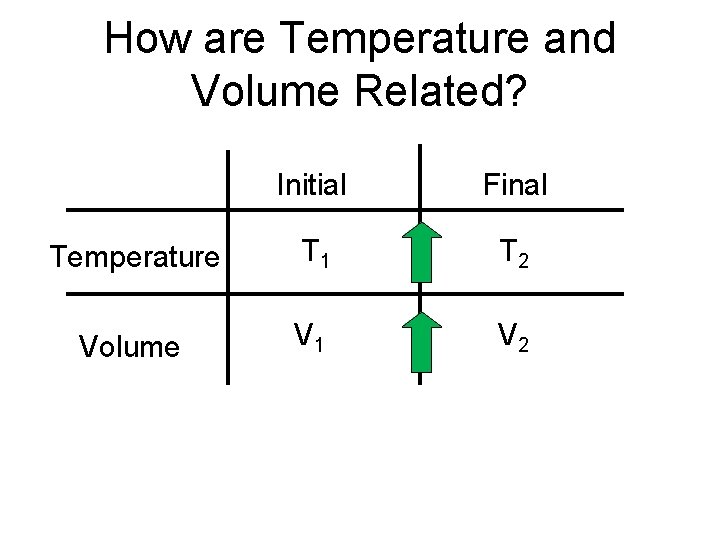
How are Temperature and Volume Related? Initial Final Temperature T 1 T 2 Volume V 1 V 2

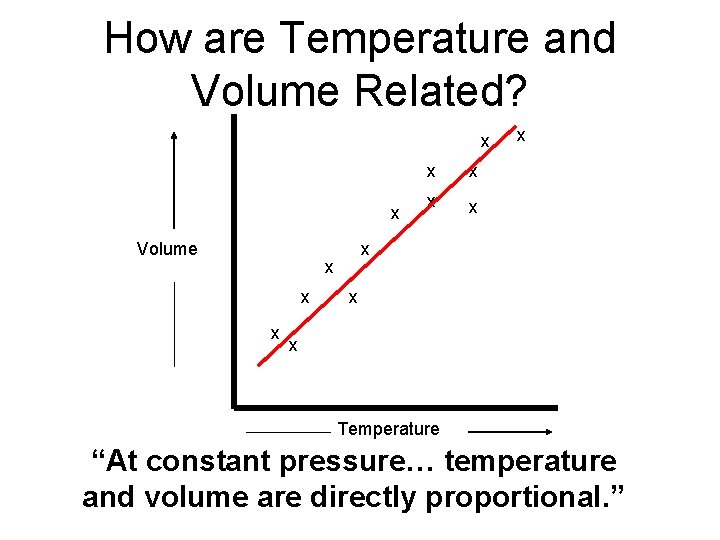
How are Temperature and Volume Related? x x Volume x x x Temperature “At constant pressure… temperature and volume are directly proportional. ”

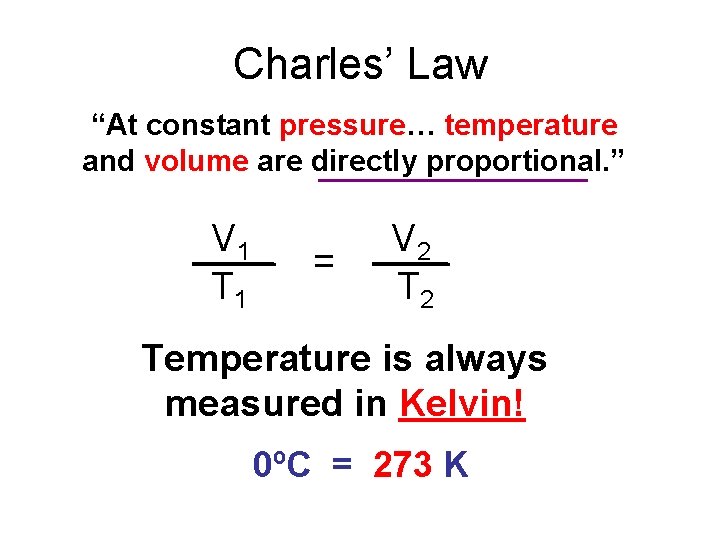
Charles’ Law “At constant pressure… temperature and volume are directly proportional. ” V 1 T 1 = V 2 Temperature is always measured in Kelvin! 0ºC = 273 K

How can we change Gases? Action Variable Heat it up/Cool it down Temperature Change container size Volume Compress or Decompress Pressure

How are Volume and Pressure Related? Initial Final Volume V 1 V 2 Pressure P 1 P 2

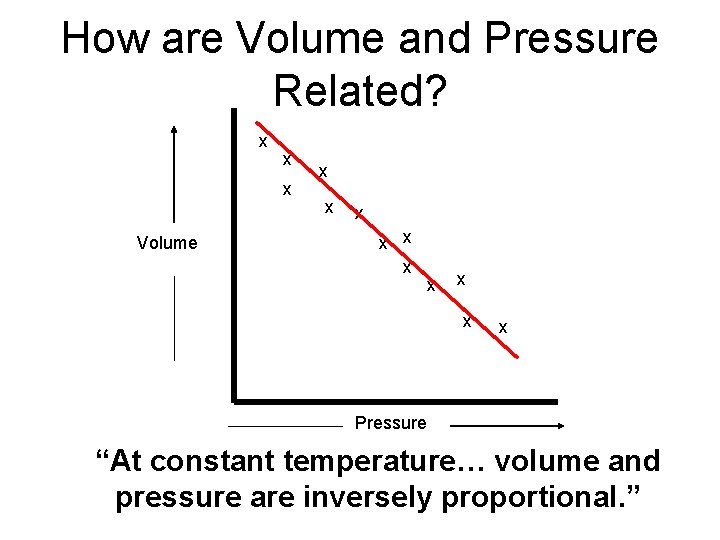
How are Volume and Pressure Related? x x x Volume x x x x x Pressure “At constant temperature… volume and pressure are inversely proportional. ”

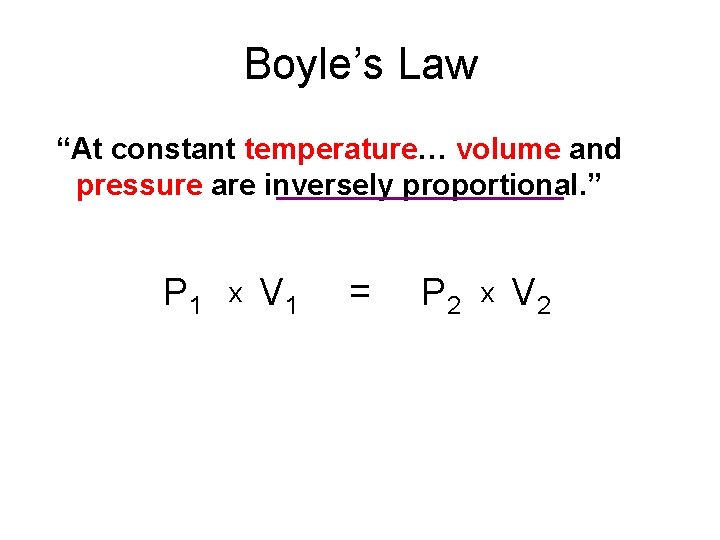
Boyle’s Law “At constant temperature… volume and pressure are inversely proportional. ” P 1 x V 1 = P 2 x V 2

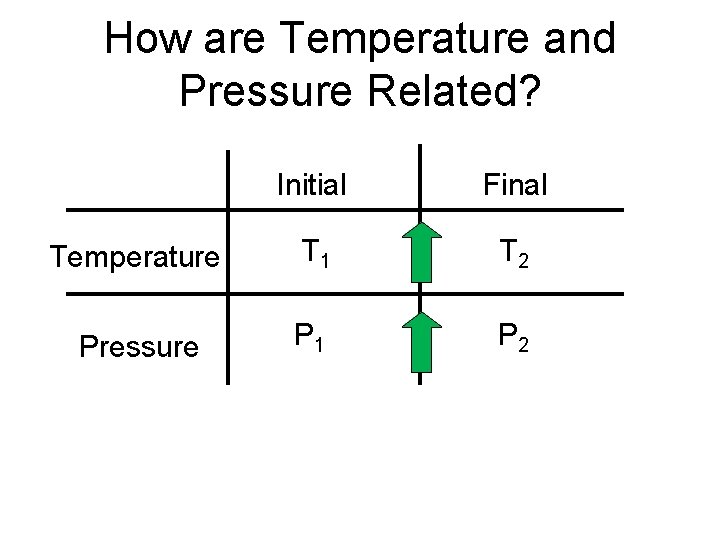
How are Temperature and Pressure Related? Initial Final Temperature T 1 T 2 Pressure P 1 P 2

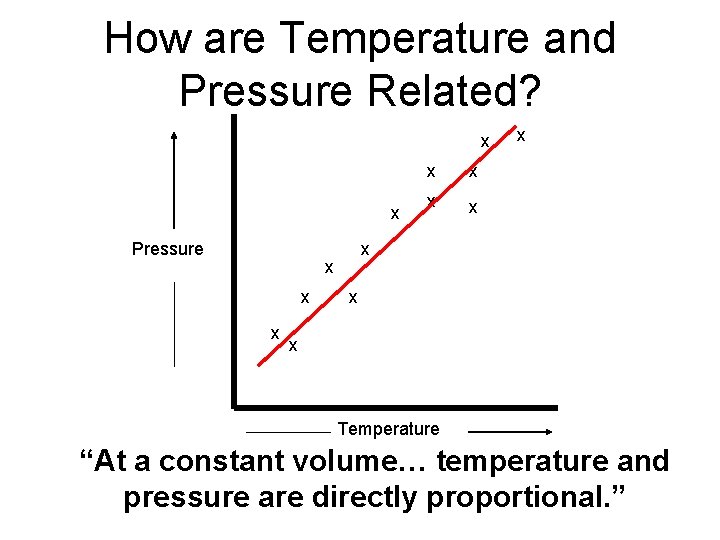
How are Temperature and Pressure Related? x x Pressure x x x Temperature “At a constant volume… temperature and pressure are directly proportional. ”

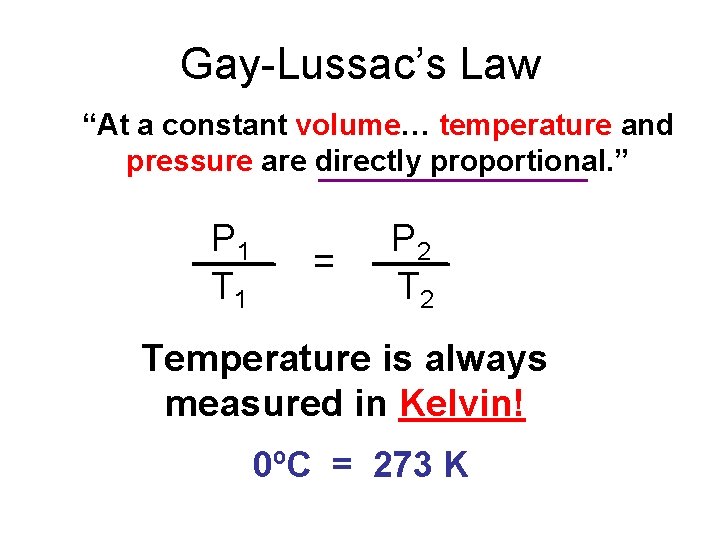
Gay-Lussac’s Law “At a constant volume… temperature and pressure are directly proportional. ” P 1 T 1 = P 2 Temperature is always measured in Kelvin! 0ºC = 273 K

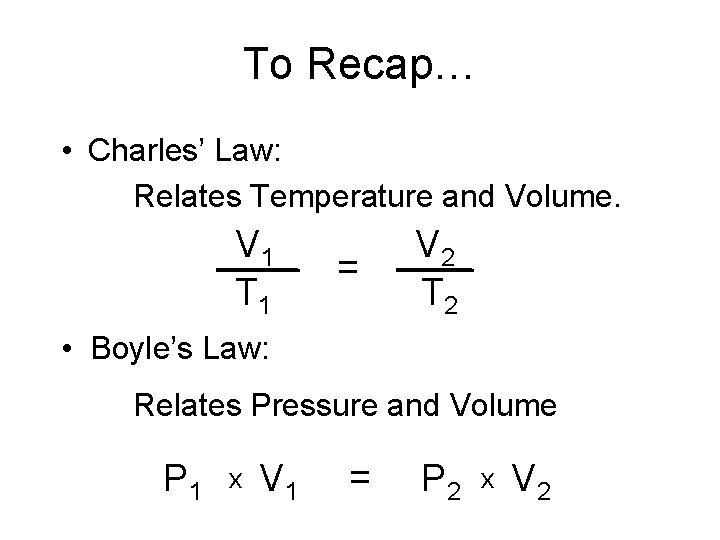
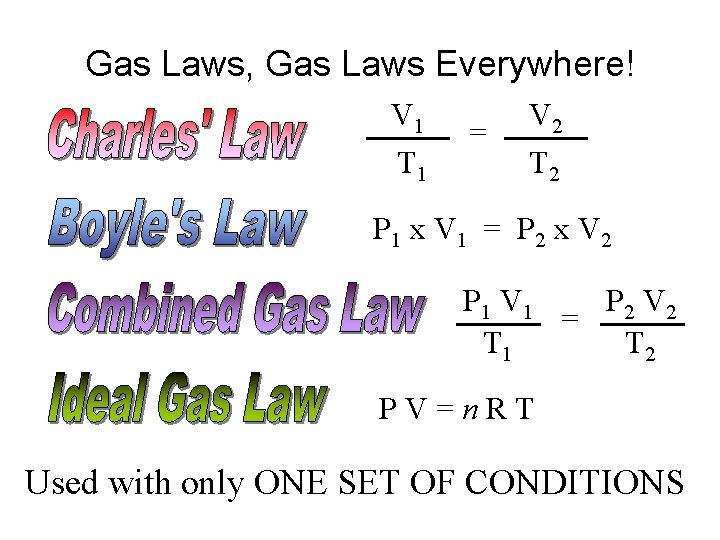
To Recap… • Charles’ Law: Relates Temperature and Volume. V 1 T 1 = V 2 T 2 • Boyle’s Law: Relates Pressure and Volume P 1 x V 1 = P 2 x V 2

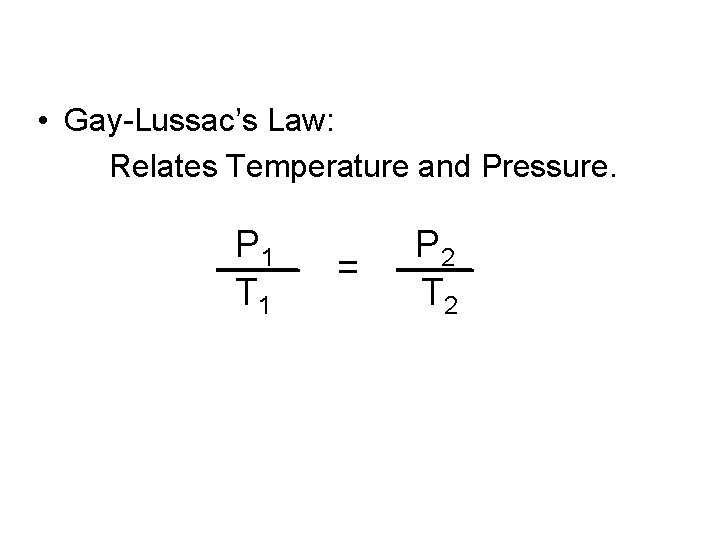
• Gay-Lussac’s Law: Relates Temperature and Pressure. P 1 T 1 = P 2 T 2

…THEREFORE: • Temperature, Volume, and Pressure all related! P 1 V 1 T 1 = P 2 V 2 T 2


A Reminder… We that we live in an where: • Gas particles have no mass world • Gas particles have no volume • Gas particles have elastic collisions These assumptions are used when trying to calculate the AMOUNT of a gas we have!

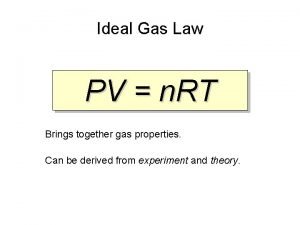
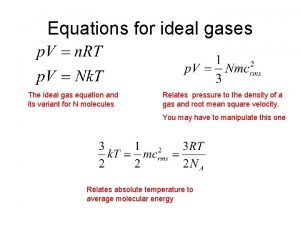
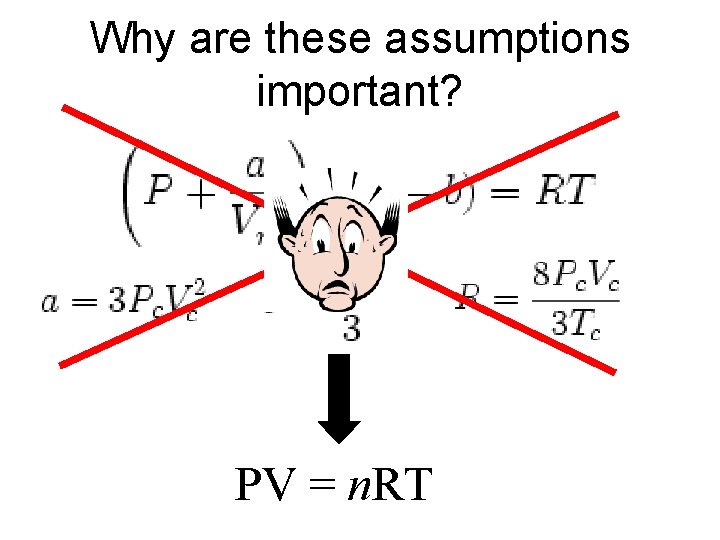
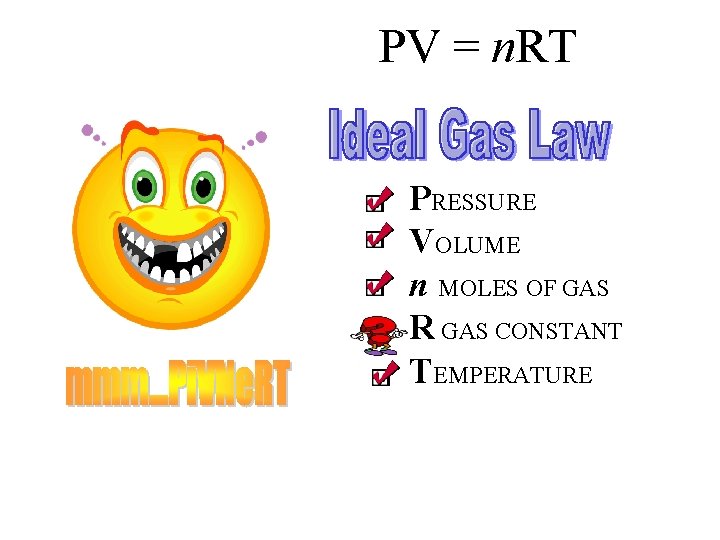
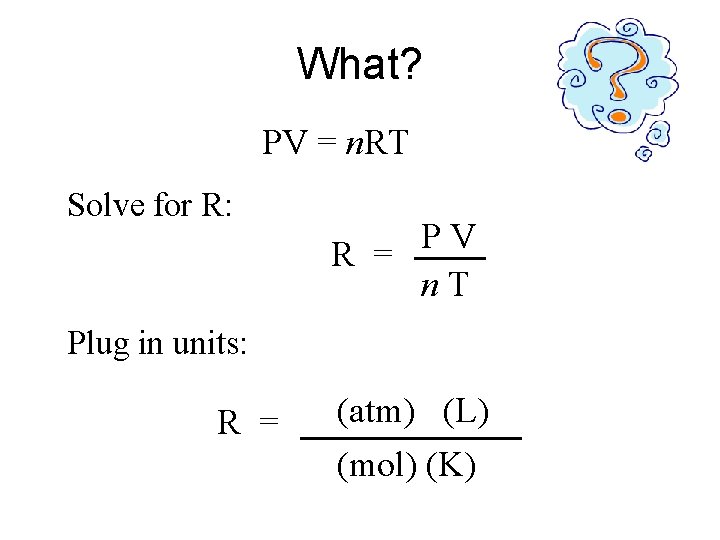
Why are these assumptions important? PV = n. RT

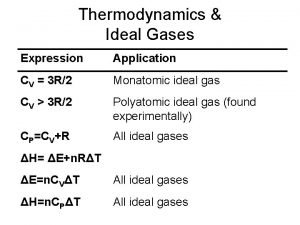
PV = n. RT PRESSURE VOLUME n MOLES OF GAS R GAS CONSTANT TEMPERATURE

The Myste. Rious R • R is a constant (doesn’t change). • Number value of R depends on other units. • Units of R are a combination of many units. 62. 4 mm. Hg · L mol · K 8. 31 k. Pa · L mol · K 0. 0821 atm · L mol · K

What? PV = n. RT Solve for R: P V R = n. T Plug in units: (k. Pa) (atm) Hg) (L) R = (mm (mol) (K)

Gas Laws, Gas Laws Everywhere! V 1 T 1 = V 2 T 2 P 1 x V 1 = P 2 x V 2 P 1 V 1 P 2 V 2 = T 1 T 2 P VCONDITIONS =n. RT Used with CHANGING Used with only ONE SET OF CONDITIONS

When to Use PV = n. RT • Calculating amount of gas in moles • Calculating P, V, or T if moles of gas are known. – IMPORTANT! We must have 3 out of 4 pieces of information: • P • V • n • T

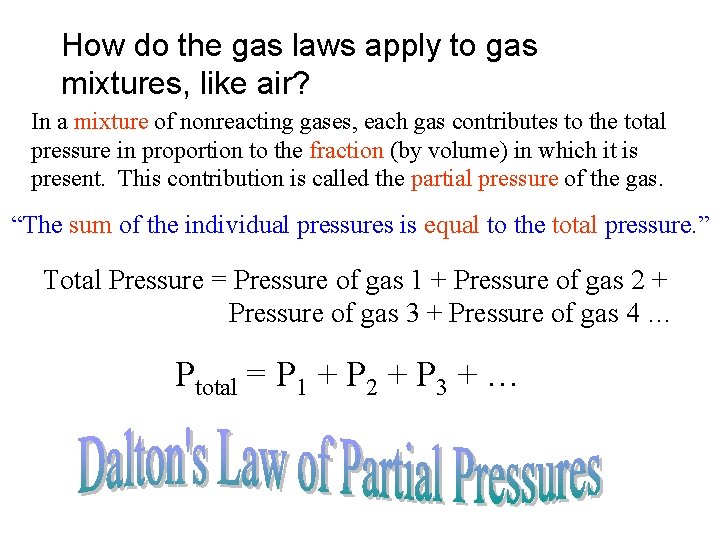
How do the gas laws apply to gas mixtures, like air? In a mixture of nonreacting gases, each gas contributes to the total pressure in proportion to the fraction (by volume) in which it is present. This contribution is called the partial pressure of the gas. “The sum of the individual pressures is equal to the total pressure. ” Total Pressure = Pressure of gas 1 + Pressure of gas 2 + Pressure of gas 3 + Pressure of gas 4 … Ptotal = P 1 + P 2 + P 3 + …

Molar Volume of a Gas Do you remember the value of one mole? 6. 02 x 1023

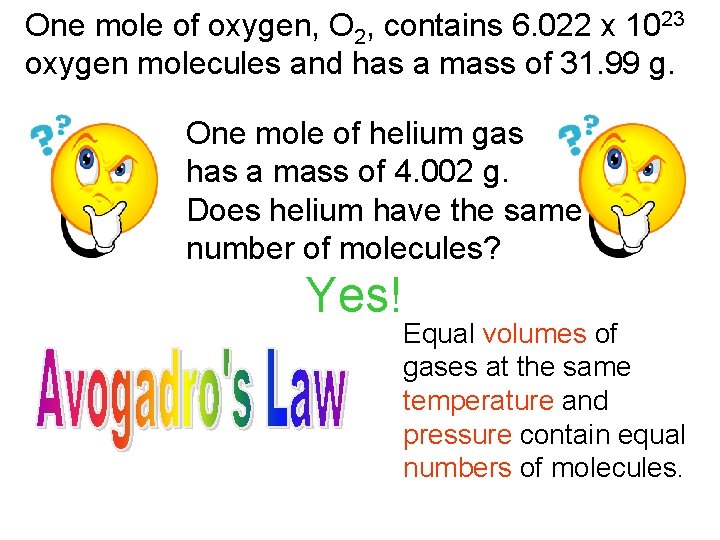
One mole of oxygen, O 2, contains 6. 022 x 1023 oxygen molecules and has a mass of 31. 99 g. One mole of helium gas has a mass of 4. 002 g. Does helium have the same number of molecules? Yes! Equal volumes of gases at the same temperature and pressure contain equal numbers of molecules.

Will 1 mole of O 2 gas and 1 mole of He gas occupy the same volume (at the same temperature and pressure) despite different masses? Remember, according to Avogadro’s law, one mole of any gas will occupy the same volume as one mole of any other gas at the same temperature and pressure, despite mass differences. The volume occupied by one mole of gas at STP. It has been found to be 22. 41410 L.
 Mikael ferm
Mikael ferm Characteristics of ideal gases
Characteristics of ideal gases Ideal gases characteristics
Ideal gases characteristics Pseudo reduced specific volume
Pseudo reduced specific volume An ideal gas is an imaginary gas
An ideal gas is an imaginary gas Differences between ideal gas and real gas
Differences between ideal gas and real gas Computational fluid dynamics
Computational fluid dynamics Difference between ideal gas and real gas
Difference between ideal gas and real gas Kinetic molecular model of gases
Kinetic molecular model of gases Compressibility of solid liquid and gas
Compressibility of solid liquid and gas First law of thermodynamics for ideal gas
First law of thermodynamics for ideal gas Are gasses highly compressible
Are gasses highly compressible Inert gases properties
Inert gases properties What are the different properties of gas?
What are the different properties of gas? Gases characteristics
Gases characteristics Physical characteristics of gases
Physical characteristics of gases Physical characteristics of gases
Physical characteristics of gases Physical characteristics of gases
Physical characteristics of gases Partial vapour pressure
Partial vapour pressure Ideal gas law with mass
Ideal gas law with mass Isothermal expansion of ideal gas
Isothermal expansion of ideal gas Monoatomic ideal gas
Monoatomic ideal gas What's an ideal gas
What's an ideal gas Charles law
Charles law Which equation agrees with the ideal gas law?
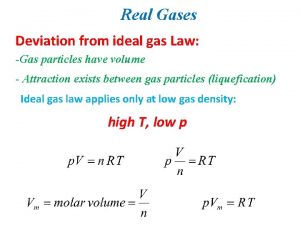
Which equation agrees with the ideal gas law? Deviation from ideal gas
Deviation from ideal gas Degree of freedom of an ideal gas
Degree of freedom of an ideal gas Pv nrt units
Pv nrt units Equation for partial pressure
Equation for partial pressure Thermodynamic behaviour of ideal bose gas
Thermodynamic behaviour of ideal bose gas Ideal gas microcanonical ensemble
Ideal gas microcanonical ensemble Deviations from the ideal gas law
Deviations from the ideal gas law R=8,31
R=8,31 Ideal gas constant mmhg
Ideal gas constant mmhg