Section 2 The Ideal Gas Law The ideal






- Slides: 20

Section 2: The Ideal Gas Law The ideal gas law relates the number of particles to pressure, temperature, and volume. K What I Know W What I Want to Find Out L What I Learned

• 9(A) Describe and calculate the relations between volume, pressure, number of moles, and temperature for an ideal gas as described by Boyle's law, Charles' law, Avogadro's law, Dalton's law of partial pressure, and the ideal gas law. • 9(C) Describe the postulates of kinetic molecular theory. • 2(I) Communicate valid conclusions supported by the data through methods such as lab reports, labeled drawings, graphs, journals, summaries, oral reports, and technology–based reports. • 3(A) In all fields of science, analyze, evaluate, and critique scientific explanations by using empirical evidence, logical reasoning, and experimental and observational testing, including examining all sides of scientific evidence of those scientific explanations, so as to encourage critical thinking by the student. Copyright © Mc. Graw-Hill Education The Ideal Gas Law

Essential Questions • How does Avogadro’s principle relate the number of particles of gas to the gas’s volume? • How is the amount of gas present related to its pressure, temperature, and volume by the ideal gas law? • What are the properties of real gases and of ideal gases? Copyright © Mc. Graw-Hill Education The Ideal Gas Law

Vocabulary Review New • mole • Avogadro’s principle • molar volume • standard temperature and pressure (STP) • ideal gas constant (R) • ideal gas law Copyright © Mc. Graw-Hill Education The Ideal Gas Law

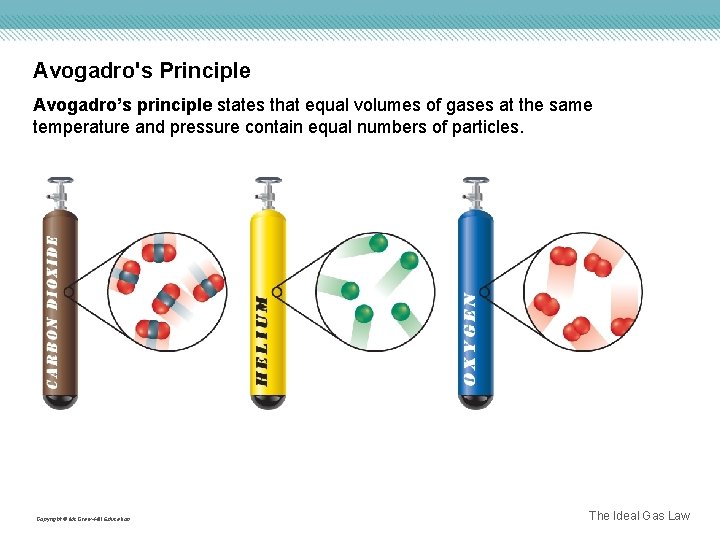
Avogadro's Principle Avogadro’s principle states that equal volumes of gases at the same temperature and pressure contain equal numbers of particles. Copyright © Mc. Graw-Hill Education The Ideal Gas Law

Avogadro's Principle The molar volume of a gas is the volume 1 mol occupies at 0. 00°C and 1. 00 atm of pressure. • 0. 00°C and 1. 00 atm are called standard temperature and pressure (STP). • At STP, 1 mol of gas occupies 22. 4 L. Copyright © Mc. Graw-Hill Education The Ideal Gas Law

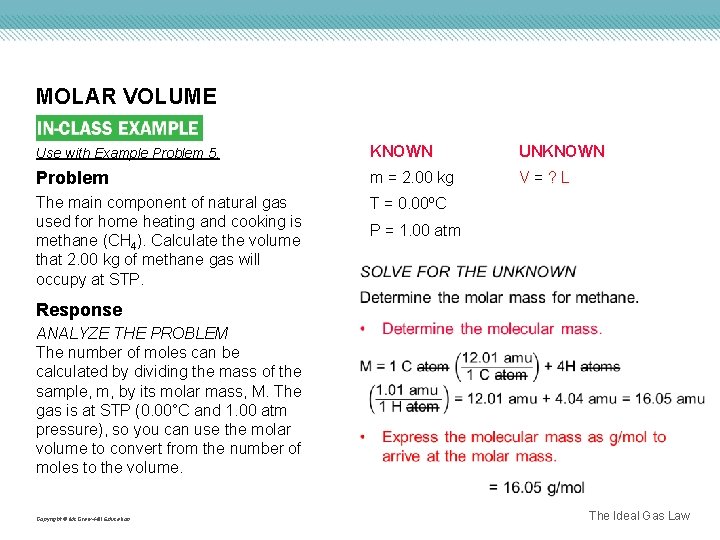
MOLAR VOLUME Use with Example Problem 5. KNOWN UNKNOWN Problem m = 2. 00 kg V=? L The main component of natural gas used for home heating and cooking is methane (CH 4). Calculate the volume that 2. 00 kg of methane gas will occupy at STP. T = 0. 00ºC P = 1. 00 atm Response ANALYZE THE PROBLEM The number of moles can be calculated by dividing the mass of the sample, m, by its molar mass, M. The gas is at STP (0. 00°C and 1. 00 atm pressure), so you can use the molar volume to convert from the number of moles to the volume. Copyright © Mc. Graw-Hill Education The Ideal Gas Law

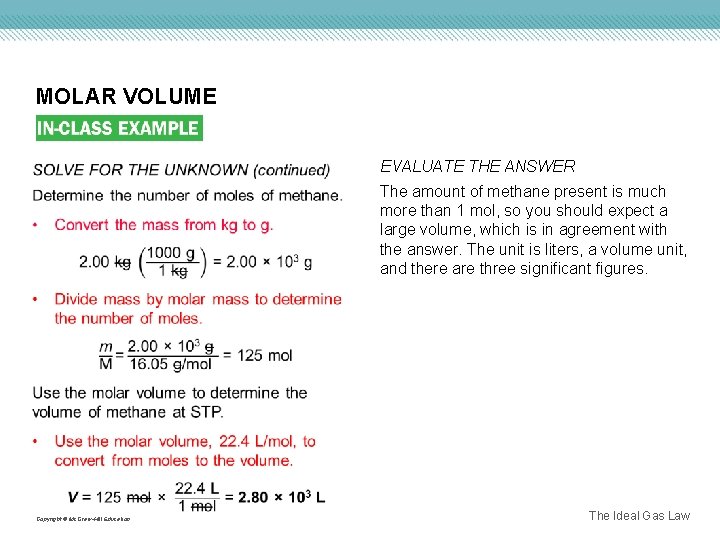
MOLAR VOLUME EVALUATE THE ANSWER The amount of methane present is much more than 1 mol, so you should expect a large volume, which is in agreement with the answer. The unit is liters, a volume unit, and there are three significant figures. Copyright © Mc. Graw-Hill Education The Ideal Gas Law

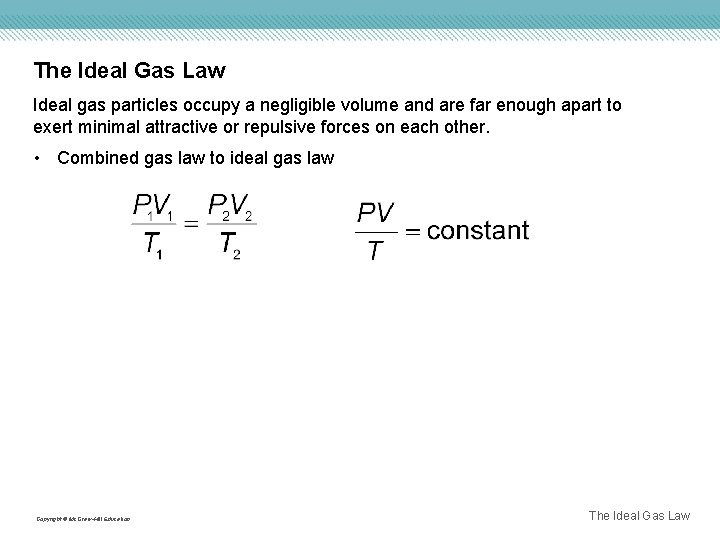
The Ideal Gas Law Ideal gas particles occupy a negligible volume and are far enough apart to exert minimal attractive or repulsive forces on each other. • Combined gas law to ideal gas law Copyright © Mc. Graw-Hill Education The Ideal Gas Law

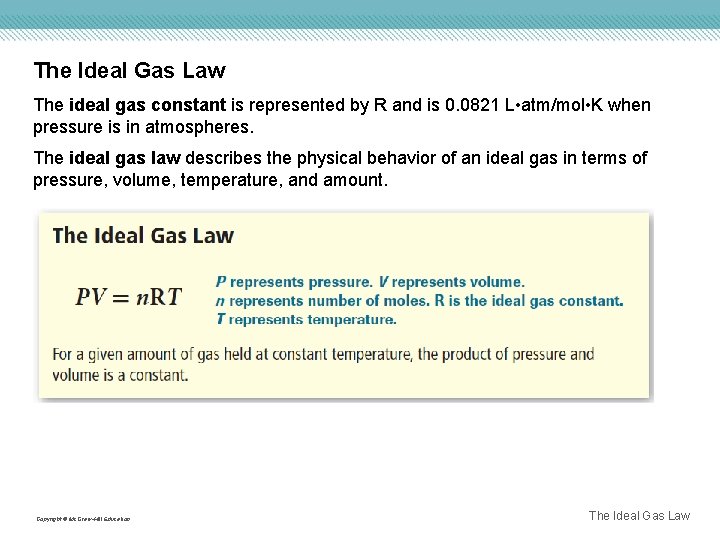
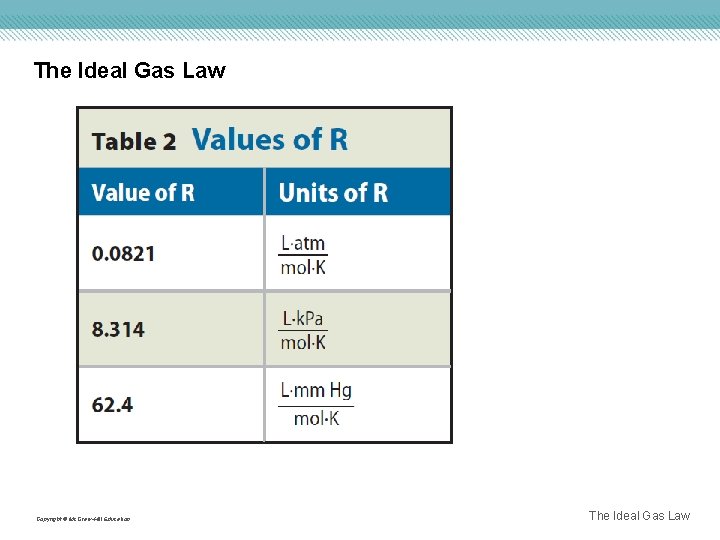
The Ideal Gas Law The ideal gas constant is represented by R and is 0. 0821 L • atm/mol • K when pressure is in atmospheres. The ideal gas law describes the physical behavior of an ideal gas in terms of pressure, volume, temperature, and amount. Copyright © Mc. Graw-Hill Education The Ideal Gas Law

The Ideal Gas Law Copyright © Mc. Graw-Hill Education The Ideal Gas Law

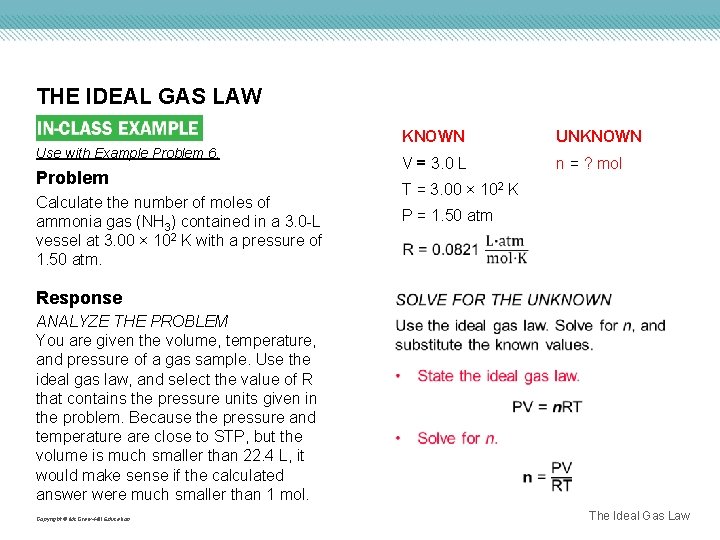
THE IDEAL GAS LAW Use with Example Problem 6. Problem Calculate the number of moles of ammonia gas (NH 3) contained in a 3. 0 -L vessel at 3. 00 × 102 K with a pressure of 1. 50 atm. Response KNOWN UNKNOWN V = 3. 0 L n = ? mol T = 3. 00 × 102 K P = 1. 50 atm ANALYZE THE PROBLEM You are given the volume, temperature, and pressure of a gas sample. Use the ideal gas law, and select the value of R that contains the pressure units given in the problem. Because the pressure and temperature are close to STP, but the volume is much smaller than 22. 4 L, it would make sense if the calculated answer were much smaller than 1 mol. Copyright © Mc. Graw-Hill Education The Ideal Gas Law

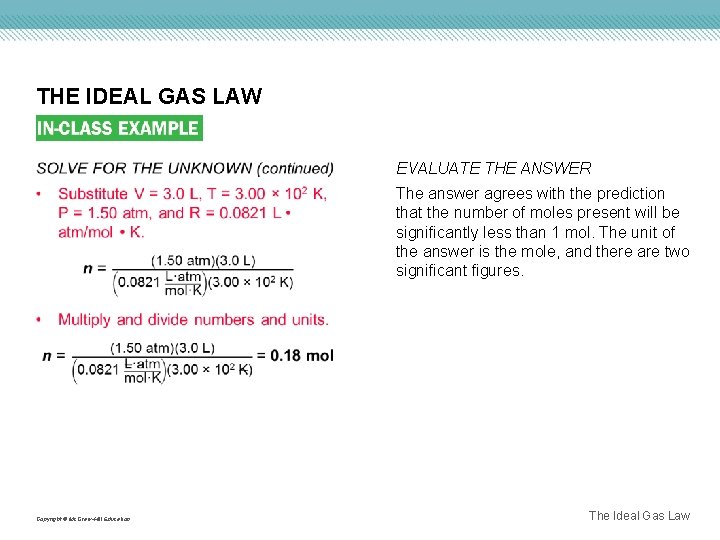
THE IDEAL GAS LAW EVALUATE THE ANSWER The answer agrees with the prediction that the number of moles present will be significantly less than 1 mol. The unit of the answer is the mole, and there are two significant figures. Copyright © Mc. Graw-Hill Education The Ideal Gas Law

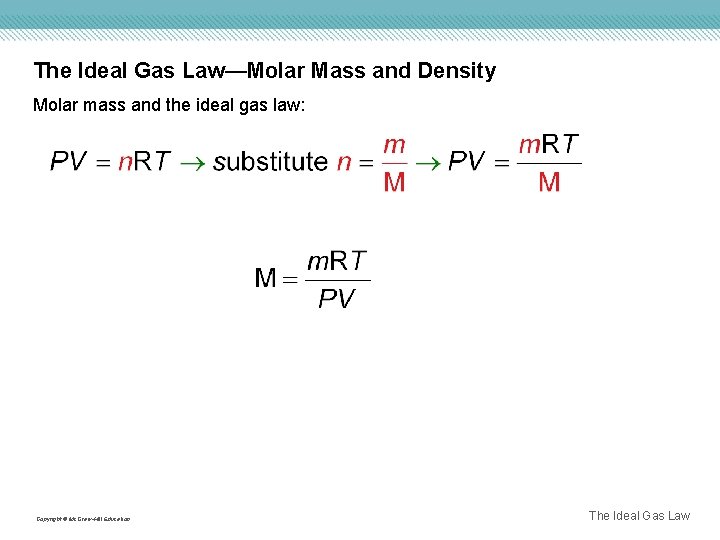
The Ideal Gas Law—Molar Mass and Density Molar mass and the ideal gas law: Copyright © Mc. Graw-Hill Education The Ideal Gas Law

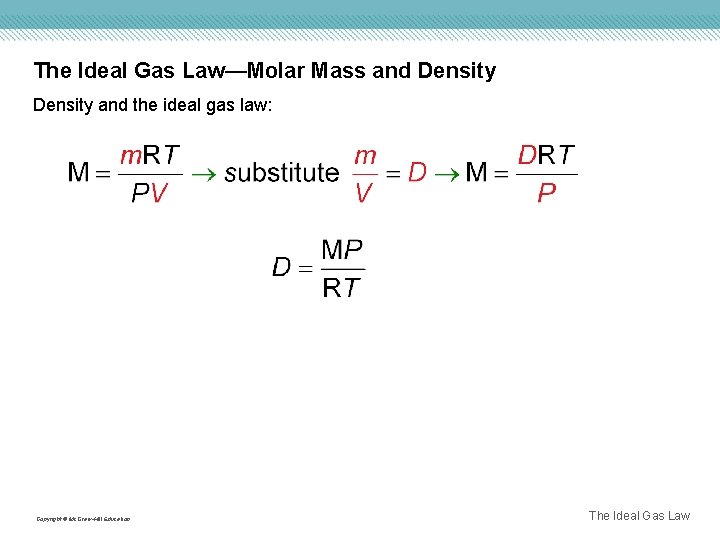
The Ideal Gas Law—Molar Mass and Density and the ideal gas law: Copyright © Mc. Graw-Hill Education The Ideal Gas Law

Gasses Video FPO Add link to video from page 456 here. Copyright © Mc. Graw-Hill Education The Ideal Gas Law

Real Versus Ideal Gases Ideal gases follow the assumptions of the kinetic-molecular theory. Characteristics of ideal gases: • There are no intermolecular attractive or repulsive forces between particles or with their containers. • The particles are in constant random motion. • Collisions are perfectly elastic. • No gas is truly ideal, but most behave as ideal gases at a wide range of temperatures and pressures. Copyright © Mc. Graw-Hill Education The Ideal Gas Law

Real Versus Ideal Gases • Real gases deviate most from ideal gases at high pressures and low temperatures. • Polar molecules have larger attractive forces between particles. • Polar gases do not behave as ideal gases. • Large nonpolar gas particles occupy more space and deviate more from ideal gases. Copyright © Mc. Graw-Hill Education The Ideal Gas Law

Gasses, Pressure and Scuba Diving Video FPO Add link to video from page 458 here. Copyright © Mc. Graw-Hill Education The Ideal Gas Law

Review Essential Questions • How does Avogadro’s principle relate the number of particles of gas to the gas’s volume? • How is the amount of gas present related to its pressure, temperature, and volume by the ideal gas law? • What are the properties of real gases and of ideal gases? Vocabulary • Avogadro’s principle • molar volume Copyright © Mc. Graw-Hill Education • standard temperature and pressure (STP) • ideal gas constant (R) • ideal gas law The Ideal Gas Law