Gas Laws For Real Gases Ideal Gases do






- Slides: 14

Gas Laws For Real Gases



Ideal Gases do not exist. But they are a useful idea. They are like real gases except: 1. Molecules are assumed to be just points in space with no length, width, or volume. 2. Molecules have no attraction with one another. They do collide and bounce off of one another. Ideal gas molecules do have mass and speed.

Real gas molecules are made up of atoms with protons, neutrons and electrons, so they do take up space and they do have electrostatic attractions for one another. So real gases deviate from the ideal gas equation, PV = n. RT, at some pressures and temperatures.

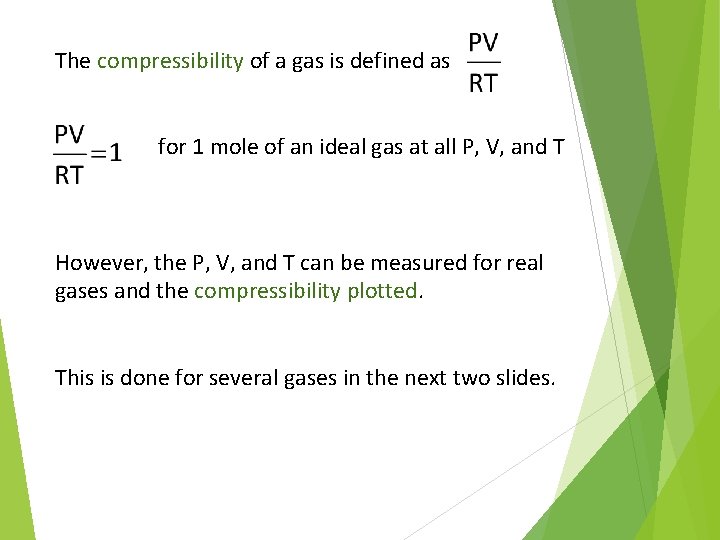
The compressibility of a gas is defined as for 1 mole of an ideal gas at all P, V, and T However, the P, V, and T can be measured for real gases and the compressibility plotted. This is done for several gases in the next two slides.

Slide 10

slide 11


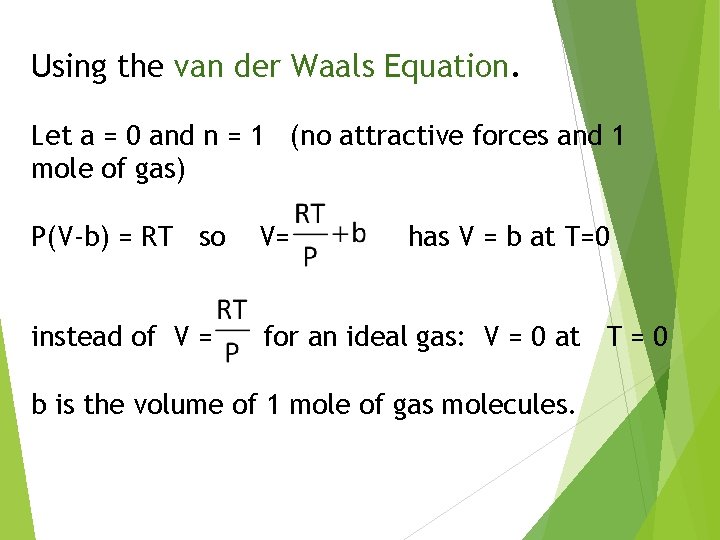
Using the van der Waals Equation. Let a = 0 and n = 1 (no attractive forces and 1 mole of gas) P(V-b) = RT so V= has V = b at T=0 instead of V = for an ideal gas: V = 0 at T = 0 b is the volume of 1 mole of gas molecules.

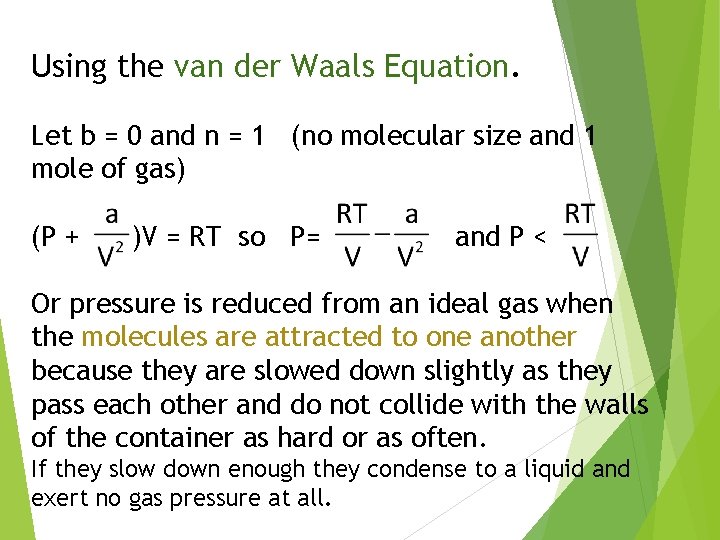
Using the van der Waals Equation. Let b = 0 and n = 1 (no molecular size and 1 mole of gas) (P + )V = RT so P= and P < Or pressure is reduced from an ideal gas when the molecules are attracted to one another because they are slowed down slightly as they pass each other and do not collide with the walls of the container as hard or as often. If they slow down enough they condense to a liquid and exert no gas pressure at all.


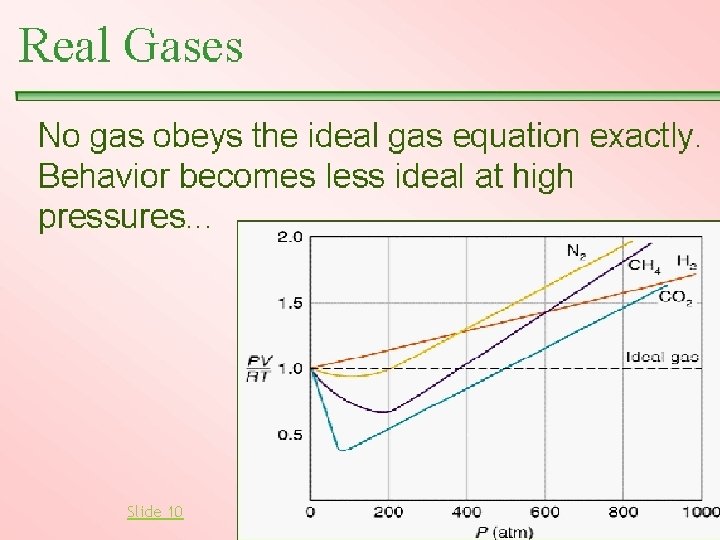
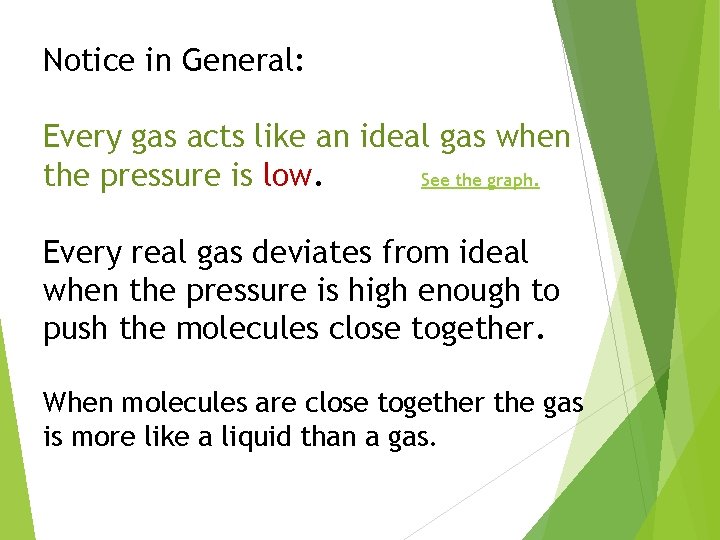
Notice in General: Every gas acts like an ideal gas when the pressure is low. See the graph. Every real gas deviates from ideal when the pressure is high enough to push the molecules close together. When molecules are close together the gas is more like a liquid than a gas.

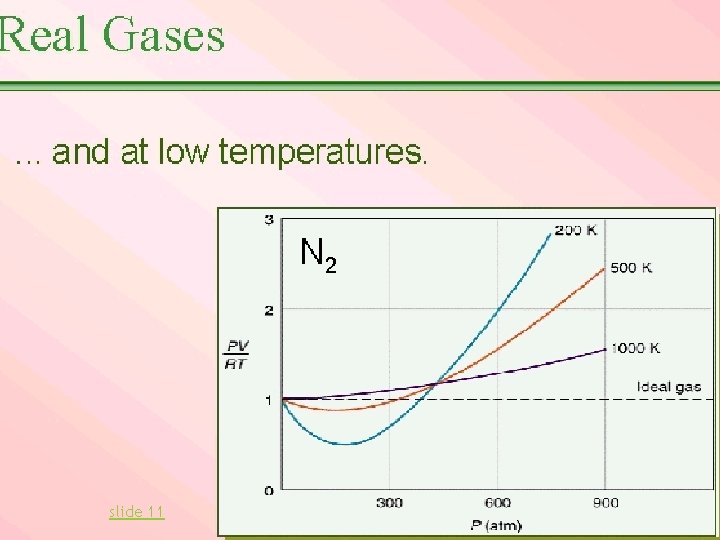
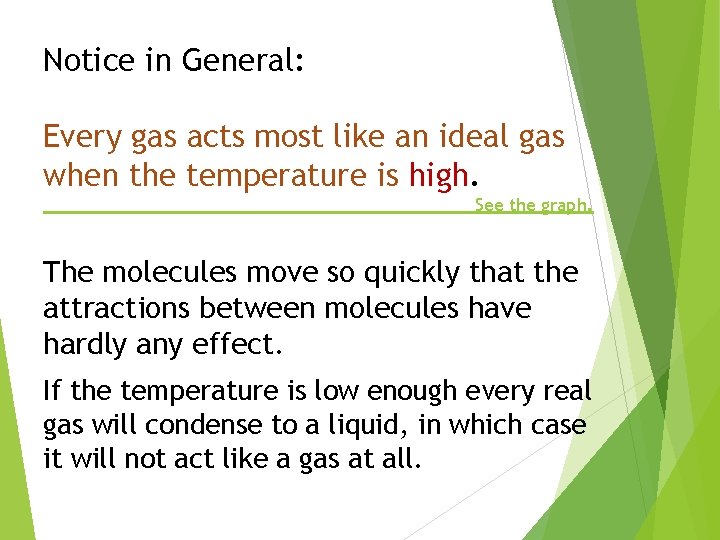
Notice in General: Every gas acts most like an ideal gas when the temperature is high. See the graph. The molecules move so quickly that the attractions between molecules have hardly any effect. If the temperature is low enough every real gas will condense to a liquid, in which case it will not act like a gas at all.
 Differences between ideal gas and real gas
Differences between ideal gas and real gas Difference between ideal gas and real gas
Difference between ideal gas and real gas Stp vs satp
Stp vs satp Unit 13
Unit 13 Derive ideal gas equation
Derive ideal gas equation Imaginary gas
Imaginary gas Computational fluid dynamics
Computational fluid dynamics Charles de secondat
Charles de secondat Kinetic molecular theory of gases
Kinetic molecular theory of gases Characteristics of ideal gases
Characteristics of ideal gases Ideal gas law characteristics
Ideal gas law characteristics Are ideal gases compressible
Are ideal gases compressible First law of thermodynamics for ideal gas
First law of thermodynamics for ideal gas Gas laws crash course
Gas laws crash course Direct or indirect relationship
Direct or indirect relationship


























