18 Heat Work First Law of Thermodynamics 1













- Slides: 26

18. Heat, Work, & First Law of Thermodynamics 熱,功,和熱力學第一定律 1. 2. 3. The 1 st Law of Thermodynamics Thermodynamic Processes Specific Heats of an Ideal Gas 熱力學第一定律 熱力學程序 理想氣體的比熱

A jet aircraft engine converts the energy of burning fuel into mechanical energy. 一具噴射機引擎把燃燒燃料所得的能量轉變成機械能。 How does energy conservation apply in this process? 如何把能量守恆用到這個程序? E combustion = E mech + Q waste E燃燒 = E機械 + Q廢棄

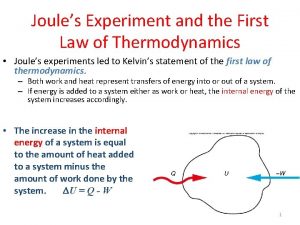
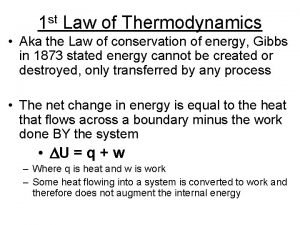
18. 1. The 1 st Law of Thermodynamics 熱力學第一定律 PE of falling weight 下墜重物的位能 KE of paddle 旋槳的動能 Heat in water 水中熱能 Either heating or stirring can raise T of the water. 加熱或攪拌都可以提升 T 。 1 st Law of Thermodynamics 熱力學第一定律: Increase in internal energy = Heat added Work done 內能增幅 = 所加熱量 所作功 Thermodynamic state variable = variable independent of history. 熱力態變數 = 跟歷程無關的變數 e. g. , U, T, P, V, … Not Q, W, … Joule’s apparatus 焦耳的儀器

Example 18. 1. Thermal Pollution 熱污染 The reactor in a nuclear power plant supplied energy at the rate of 3. 0 GW, 一個核電廠的反應爐以 3. 0 GW 的功率供應能量, boiling water to produce steam that turns a turbine-generator. 其間先把水燒成蒸氣,再以之推動一部渦輪發電機。 The spent steam is then condensed through thermal contact with water taken from a river. 用完的蒸氣則與一條河的水經過熱接觸後凝結。 If the power plant produces electrical energy at the rate of 1. 0 GW, at what rate is heat transferred to the river? 如果這發電廠以 1. 0 GW 的功率供應電能,熱傳到河裏的功率是多少? 1 st law 第一定律 From standpoint of power plant: 以核電廠的角度來看: ( loses heat to river ) 流失到河裏

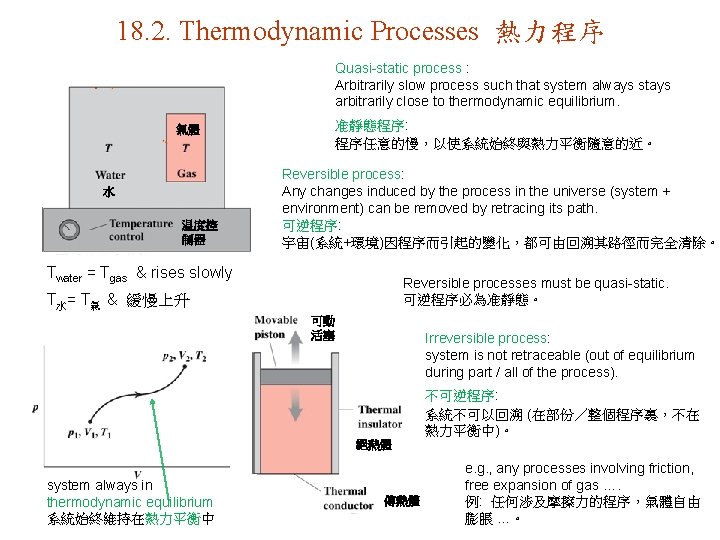
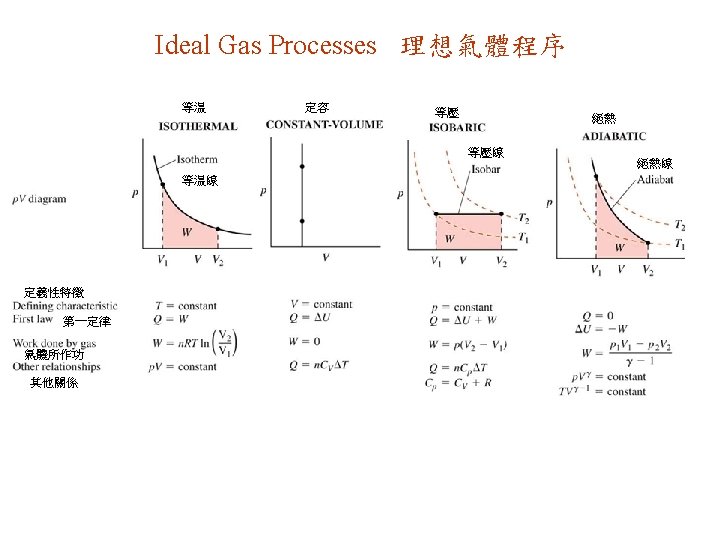
18. 2. Thermodynamic Processes 熱力程序 Quasi-static process : Arbitrarily slow process such that system always stays arbitrarily close to thermodynamic equilibrium. 准靜態程序: 程序任意的慢,以使系統始終與熱力平衡隨意的近。 氣體 水 温度控 制器 Reversible process: Any changes induced by the process in the universe (system + environment) can be removed by retracing its path. 可逆程序: 宇宙(系統+環境)因程序而引起的變化,都可由回溯其路徑而完全清除。 Twater = Tgas & rises slowly Reversible processes must be quasi-static. 可逆程序必為准靜態。 T水= T氣 & 緩慢上升 可動 活塞 Irreversible process: system is not retraceable (out of equilibrium during part / all of the process). 不可逆程序: 系統不可以回溯 (在部份/整個程序裏,不在 熱力平衡中)。 絕熱體 system always in thermodynamic equilibrium 系統始終維持在熱力平衡中 傳熱體 e. g. , any processes involving friction, free expansion of gas …. 例: 任何涉及摩擦力的程序,氣體自由 膨脹 …。

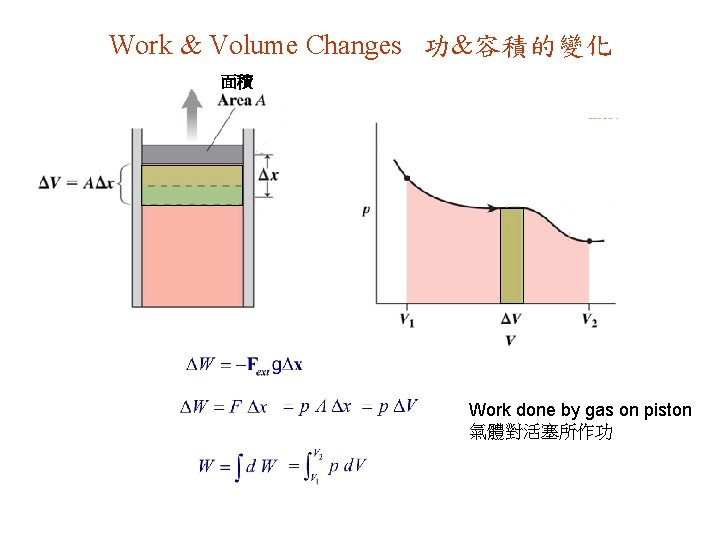
Work & Volume Changes 功&容積的變化 面積 Work done by gas on piston 氣體對活塞所作功

GOT IT 懂嗎 ? 18. 1. Two identical gas-cylinder systems are taken from the same initial state to the same final state, but by different processes. 兩個一樣的汽缸系統,從同一個初態,經由不同的程序,達到同一個終態。 Which are the same in both cases 下面那一項在兩者都一樣: (a) the work done on or by the gas, 氣體所作或被作的功, (b) the heat added or removed, or 加入或移除的熱,或 (c) the change in internal energy? 內能的變化?

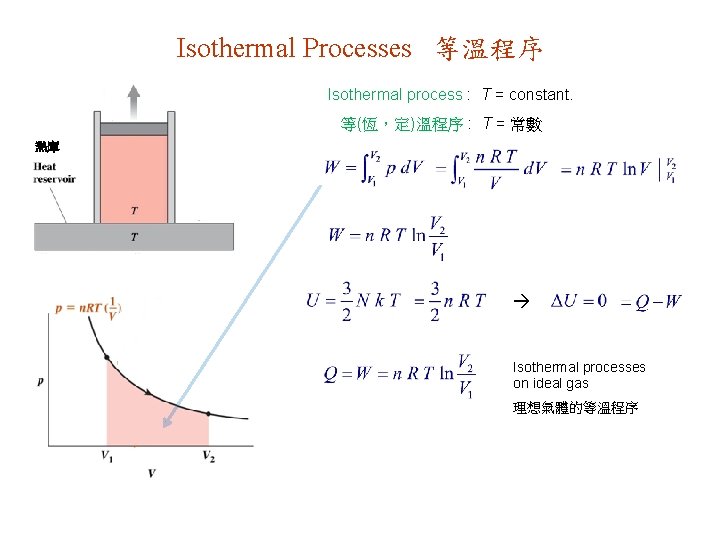
Isothermal Processes 等溫程序 Isothermal process : T = constant. 等(恆,定)溫程序 : T = 常數 熱庫 Isothermal processes on ideal gas 理想氣體的等溫程序

Example 18. 2. Bubbles 氣泡 ! A scuba diver is 25 m down, where the pressure is 3. 5 atm ( 350 k. Pa ). 一個潛水員在 25 m 水深處,壓力為 3. 5 atm ( 350 k. Pa ) 。 The air she exhales forms bubbles 8. 0 mm in radius. 她呼出的空氣形成半徑為 8. 0 mm 的氣泡。 How much work does each bubble do as it arises to the surface, assuming the bubbles remain at 300 K. 每個氣泡升到水面時作功多少? 假定氣泡保持在 300 K。 T = const

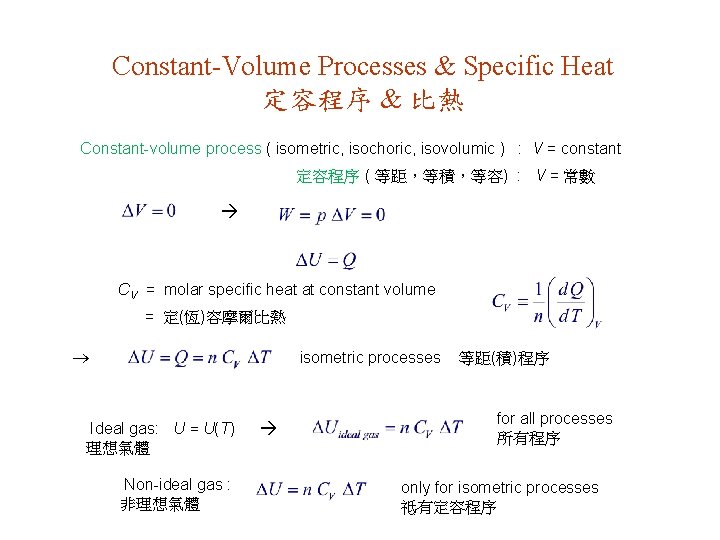
Constant-Volume Processes & Specific Heat 定容程序 & 比熱 Constant-volume process ( isometric, isochoric, isovolumic ) : V = constant 定容程序 ( 等距,等積,等容) : V = 常數 CV = molar specific heat at constant volume = 定(恆)容摩爾比熱 isometric processes Ideal gas: U = U(T) 理想氣體 Non-ideal gas : 非理想氣體 等距(積)程序 for all processes 所有程序 only for isometric processes 祗有定容程序

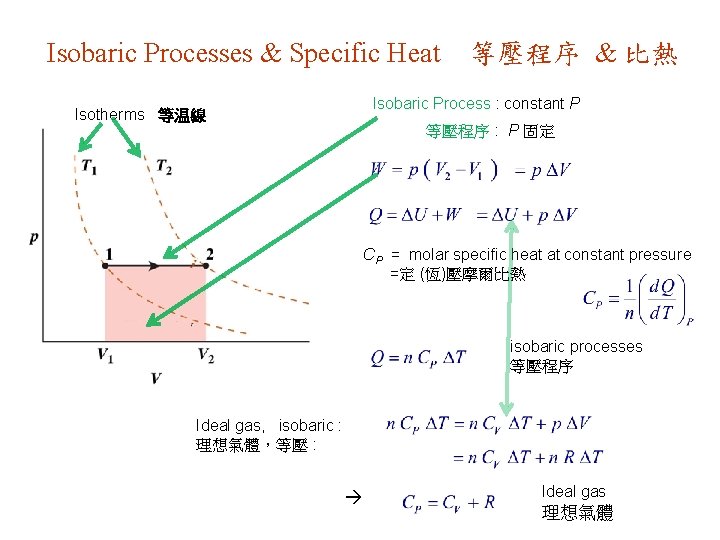
Isobaric Processes & Specific Heat 等壓程序 & 比熱 Isobaric Process : constant P Isotherms 等温線 等壓程序 : P 固定 CP = molar specific heat at constant pressure =定 (恆)壓摩爾比熱 isobaric processes 等壓程序 Ideal gas, isobaric : 理想氣體,等壓 : Ideal gas 理想氣體

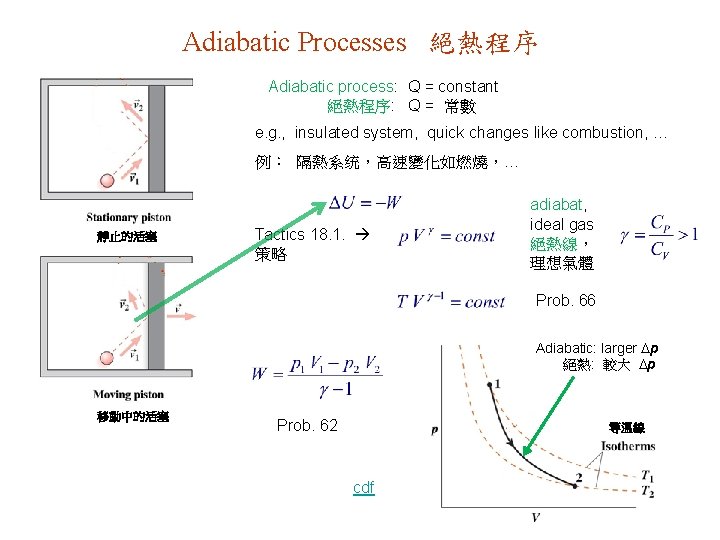
Adiabatic Processes 絕熱程序 Adiabatic process: Q = constant 絕熱程序: Q = 常數 e. g. , insulated system, quick changes like combustion, … 例: 隔熱系统,高速變化如燃燒,… 靜止的活塞 Tactics 18. 1. 策略 adiabat, ideal gas 絕熱線, 理想氣體 Prob. 66 Adiabatic: larger p 絕熱: 較大 p 移動中的活塞 Prob. 62 等温線 cdf

TACTIC 18. 1. Adiabatic Equation 絕熱方程 Ideal gas, any process: 理想氣體,任何程序: Adiabatic process: 絕熱程序:

Conceptual Example 18. 1. Ideal-Gas Law vs Adiabatic Equation 理想氣體定律對絕熱方程 The ideal gas law says p V = n R T, 理想氣體定律說 p V = n R T but the adiabatic equation says p V = const. 絕熱方程卻說 p V = const. Which is right ? 那一個對? Both are right. 都對。 The adiabatic equation is a special case where T V +1 絕熱方程是個在 T V +1 時的特例。

Making the Connection Suppose you halve the volume of an ideal gas with = 1. 4. What happens to the pressure if the process is (a) isothermal and (b) adiabatic? Ans: (a) p. V = const p = 2 p 0 (doubles) (b) p V = const p = p 0 2 = 2. 64 p 0

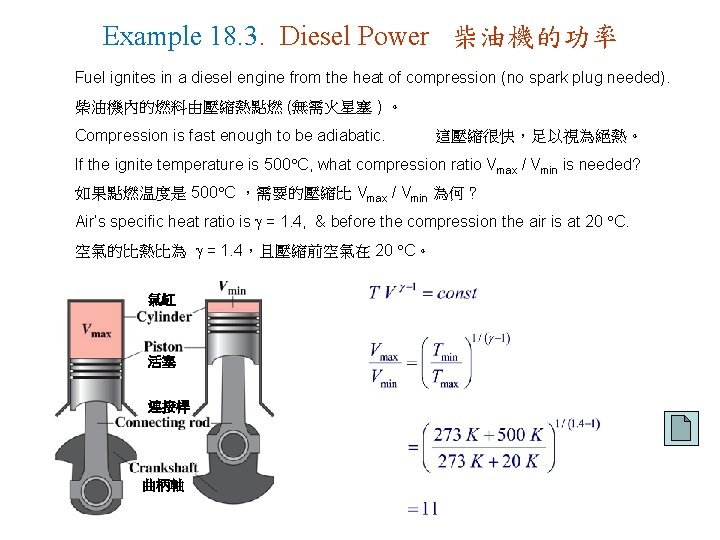
Example 18. 3. Diesel Power 柴油機的功率 Fuel ignites in a diesel engine from the heat of compression (no spark plug needed). 柴油機內的燃料由壓縮熱點燃 (無需火星塞 ) 。 Compression is fast enough to be adiabatic. 這壓縮很快,足以視為絕熱。 If the ignite temperature is 500 C, what compression ratio Vmax / Vmin is needed? 如果點燃温度是 500 C ,需要的壓縮比 Vmax / Vmin 為何? Air’s specific heat ratio is = 1. 4, & before the compression the air is at 20 C. 空氣的比熱比為 = 1. 4,且壓縮前空氣在 20 C。 氣缸 活塞 連接桿 曲柄軸

Application: Smog Alert! 應用: 煙霧警報! Air is poor heat conductor convection is adiabatic. 空氣是不良熱導體 對流是絕熱程序。 If rising air cools slower than surrounding air, 如果上升空氣的冷卻比周邊的空氣慢, pollution rises high & can be dispersed. 汚染物就可以升很高而散掉。 Otherwise, smog. 反之,則成煙霧。

GOT IT 懂嗎 ? 18. 2. Name the basic thermodynamic process involved when each of the following is done to a piston-cylinder system containing ideal gas, 點出下列每項作用在一個理想氣體汽缸系統時,所牽涉的熱力程序, & tell also whether T, p, V, & U increase or decrease. 也說出 T, p, V, & U 是增加還是減少。 (a) the piston is lock in place & a flame is applied to the bottom of the cylinder, 活塞位置鎖定,汽缸底部以火燒之, (b) the cylinder is completely insulated & the piston is pushed downward, 汽缸完全隔熱,活塞往下推, (c) the piston is exposed to atmospheric pressure & is free to move, while the cylinder is cooled by placing it on a block of ice. 活塞外通大氣壓且可以自由移動,汽缸則放在一塊冰上冷卻。 (a) isometric 等容; T , p , (b) adiabatic 絕熱 ; T , p , V =const, U . V , U . (c) isobaric 等壓 ; T , p =const, V , U .


Cyclic Processes 循還程序 Cyclic Process : system returns to same thermodynamic state periodically. 循還程序 : 系统週期性地回到同一個熱力態。

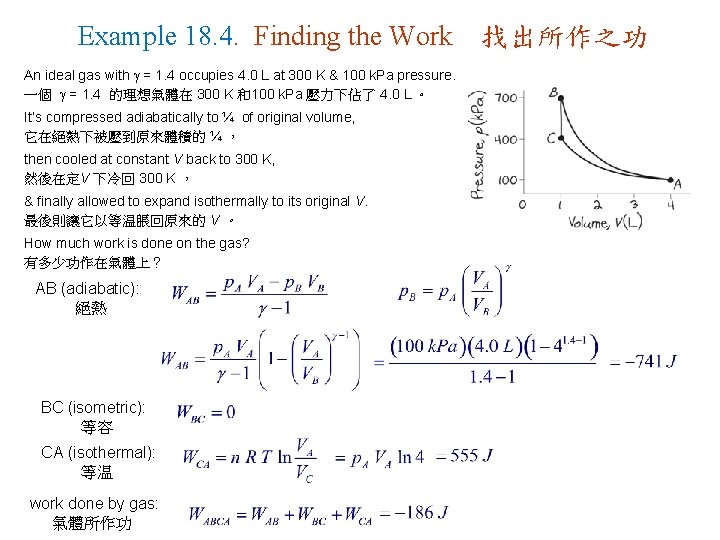
Example 18. 4. Finding the Work An ideal gas with = 1. 4 occupies 4. 0 L at 300 K & 100 k. Pa pressure. 一個 = 1. 4 的理想氣體在 300 K 和100 k. Pa 壓力下佔了 4. 0 L 。 It’s compressed adiabatically to ¼ of original volume, 它在絕熱下被壓到原來體積的 ¼ , then cooled at constant V back to 300 K, 然後在定V 下冷回 300 K , & finally allowed to expand isothermally to its original V. 最後則讓它以等温脹回原來的 V 。 How much work is done on the gas? 有多少功作在氣體上? AB (adiabatic): 絕熱 BC (isometric): 等容 CA (isothermal): 等温 work done by gas: 氣體所作功 找出所作之功

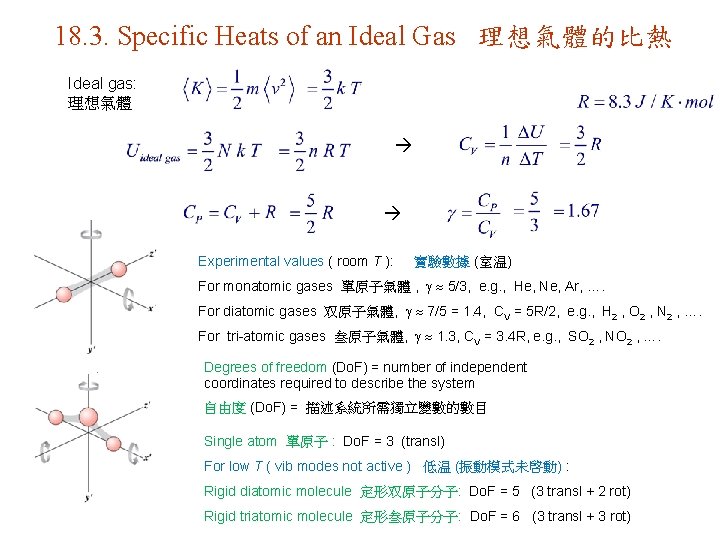
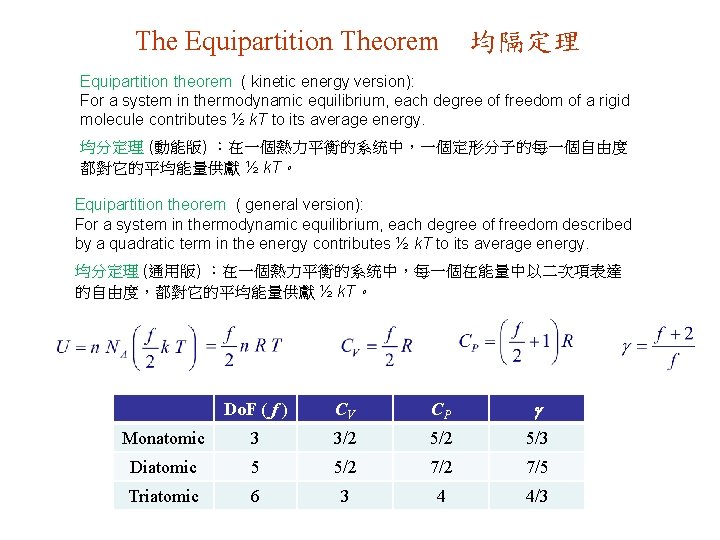
18. 3. Specific Heats of an Ideal Gas 理想氣體的比熱 Ideal gas: 理想氣體 Experimental values ( room T ): 實驗數據 (室温) For monatomic gases 單原子氣體 , 5/3, e. g. , He, Ne, Ar, …. For diatomic gases 双原子氣體, 7/5 = 1. 4, CV = 5 R/2, e. g. , H 2 , O 2 , N 2 , …. For tri-atomic gases 叁原子氣體, 1. 3, CV = 3. 4 R, e. g. , SO 2 , NO 2 , …. Degrees of freedom (Do. F) = number of independent coordinates required to describe the system 自由度 (Do. F) = 描述系統所需獨立變數的數目 Single atom 單原子 : Do. F = 3 (transl) For low T ( vib modes not active ) 低温 (振動模式未啓動) : Rigid diatomic molecule 定形双原子分子: Do. F = 5 (3 transl + 2 rot) Rigid triatomic molecule 定形叁原子分子: Do. F = 6 (3 transl + 3 rot)

The Equipartition Theorem 均隔定理 Equipartition theorem ( kinetic energy version): For a system in thermodynamic equilibrium, each degree of freedom of a rigid molecule contributes ½ k. T to its average energy. 均分定理 (動能版) :在一個熱力平衡的系统中,一個定形分子的每一個自由度 都對它的平均能量供獻 ½ k. T。 Equipartition theorem ( general version): For a system in thermodynamic equilibrium, each degree of freedom described by a quadratic term in the energy contributes ½ k. T to its average energy. 均分定理 (通用版) :在一個熱力平衡的系统中,每一個在能量中以二次項表達 的自由度,都對它的平均能量供獻 ½ k. T。 Do. F ( f ) CV CP Monatomic 3 3/2 5/3 Diatomic 5 5/2 7/5 Triatomic 6 3 4 4/3

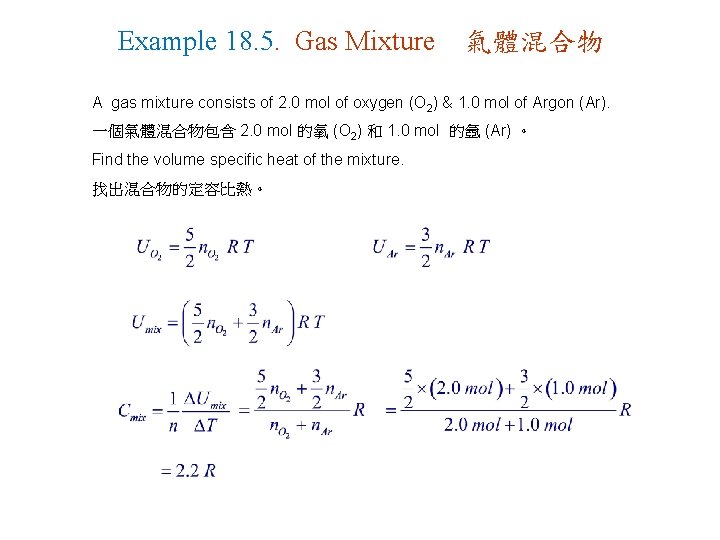
Example 18. 5. Gas Mixture 氣體混合物 A gas mixture consists of 2. 0 mol of oxygen (O 2) & 1. 0 mol of Argon (Ar). 一個氣體混合物包含 2. 0 mol 的氧 (O 2) 和 1. 0 mol 的氬 (Ar) 。 Find the volume specific heat of the mixture. 找出混合物的定容比熱。

Quantum Effects 量子効應 Quantum effect: 量子効應: Each mechanism has a threshold energy. 每個機制都有一個能量門檻。 Etransl < Erot < Evib E平移 < E轉動 < E振動 Translation+rotation+vibration 定 積 比 熱 平移+轉動+振動 Translation + rotation 平移 + 轉動 Translation 平移 温度 CV of H 2 gas as function of T. H 2 氣體的 CV 隨 T 的變化。 Below 20 K hydrogen is liquid, 20 K 以下氫是液體, above 3200 K it dissociates into individual atoms. 3200 K 以上它分解成個別原子。

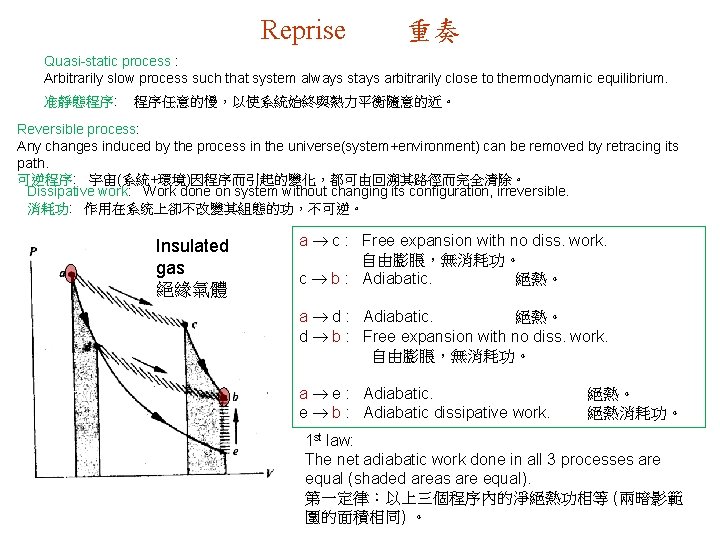
Reprise 重奏 Quasi-static process : Arbitrarily slow process such that system always stays arbitrarily close to thermodynamic equilibrium. 准靜態程序: 程序任意的慢,以使系統始終與熱力平衡隨意的近。 Reversible process: Any changes induced by the process in the universe(system+environment) can be removed by retracing its path. 可逆程序: 宇宙(系統+環境)因程序而引起的變化,都可由回溯其路徑而完全清除。 Dissipative work: Work done on system without changing its configuration, irreversible. 消耗功: 作用在系统上卻不改變其組態的功,不可逆。 Insulated gas 絕緣氣體 a c : Free expansion with no diss. work. 自由膨脹,無消耗功。 c b : Adiabatic. 絕熱。 a d : Adiabatic. 絕熱。 d b : Free expansion with no diss. work. 自由膨脹,無消耗功。 a e : Adiabatic. e b : Adiabatic dissipative work. 絕熱。 絕熱消耗功。 1 st law: The net adiabatic work done in all 3 processes are equal (shaded areas are equal). 第一定律:以上三個程序內的淨絕熱功相等 (兩暗影範 圍的面積相同) 。
 Steady flow energy equation thermodynamics
Steady flow energy equation thermodynamics Isobaric process formula
Isobaric process formula First law of thermodynamics cyclic process
First law of thermodynamics cyclic process Joule's first law
Joule's first law First law of thermodynamics
First law of thermodynamics Steady flow process in thermodynamics
Steady flow process in thermodynamics First law of thermodynamics sign convention
First law of thermodynamics sign convention Enthalpy vs internal energy
Enthalpy vs internal energy First law of thermodynamics
First law of thermodynamics Thermodynamics of ideal gases
Thermodynamics of ideal gases Newton's first law and second law and third law
Newton's first law and second law and third law Newton's first law of motion
Newton's first law of motion Dr erukhimova
Dr erukhimova What is flow work in thermodynamics
What is flow work in thermodynamics Zeroth law of thermodynamics
Zeroth law of thermodynamics Third law of thermodynamics derivation
Third law of thermodynamics derivation Third law of thermodynamics
Third law of thermodynamics Formula for entropy change
Formula for entropy change Zeroth law of thermodynamics
Zeroth law of thermodynamics Second law of thermodynamics
Second law of thermodynamics Laws of thermodynamics
Laws of thermodynamics Second law of thermodynamics
Second law of thermodynamics Second law of thermodynamics
Second law of thermodynamics Thermodynamics enthalpy of reaction and hess's law
Thermodynamics enthalpy of reaction and hess's law Third law of thermodynamics is depend on
Third law of thermodynamics is depend on Dq=cdt
Dq=cdt 1st law of thermodynamics
1st law of thermodynamics