The differences between ideal gas and real gas
The differences between ideal gas and real gas model part no. 1: HP simulations Przemysław Smakulski 26 -04 -2018

After meeting in GSI
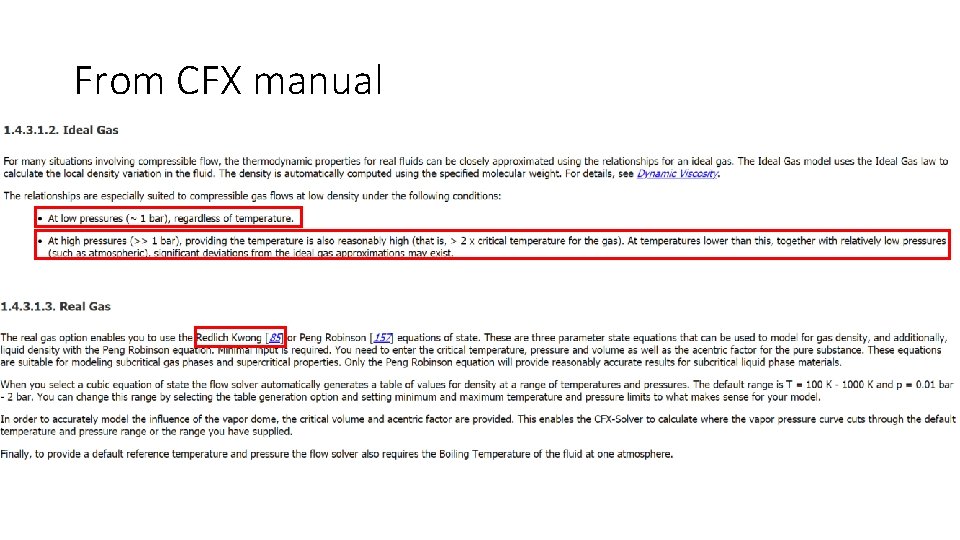
From CFX manual
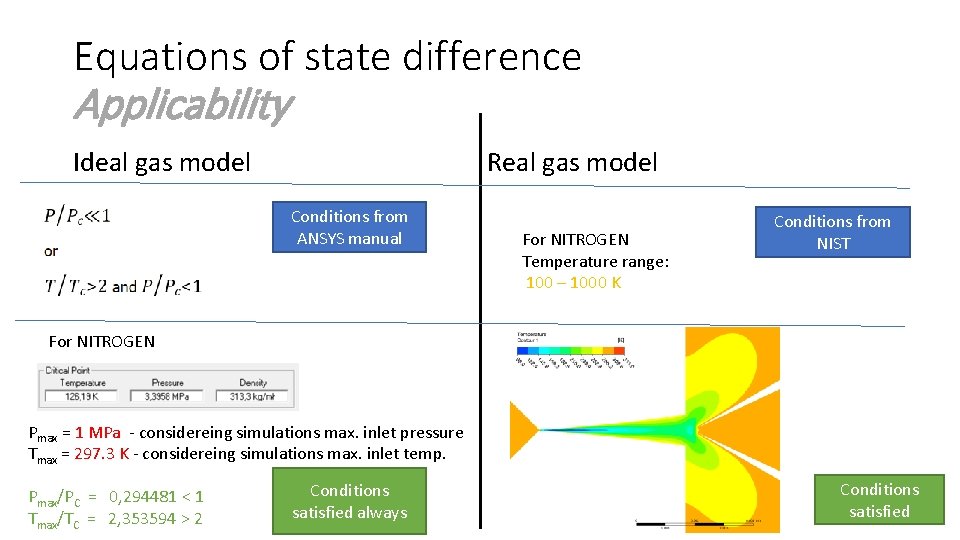
Equations of state difference Applicability Ideal gas model Real gas model Conditions from ANSYS manual For NITROGEN Temperature range: 100 – 1000 K Conditions from NIST For NITROGEN Pmax = 1 MPa - considereing simulations max. inlet pressure Tmax = 297. 3 K - considereing simulations max. inlet temp. Pmax/PC = 0, 294481 < 1 Tmax/TC = 2, 353594 > 2 Conditions satisfied always Conditions satisfied
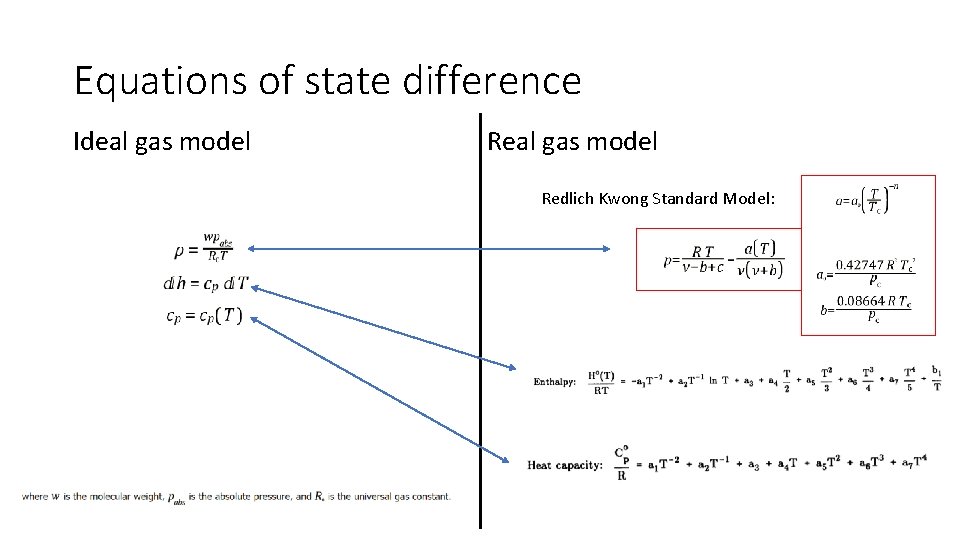
Equations of state difference Ideal gas model Redlich Kwong Standard Model:
![Real Gas properties NITROGEN [1] B. J. Mc. Bride, S. Gordon, M. a. Reno, Real Gas properties NITROGEN [1] B. J. Mc. Bride, S. Gordon, M. a. Reno,](http://slidetodoc.com/presentation_image_h2/7653f00a8f4c41e148590fe8dcbaa3d1/image-6.jpg)
Real Gas properties NITROGEN [1] B. J. Mc. Bride, S. Gordon, M. a. Reno, Thermodynamic Data for Fifty Reference Elements, (1993).
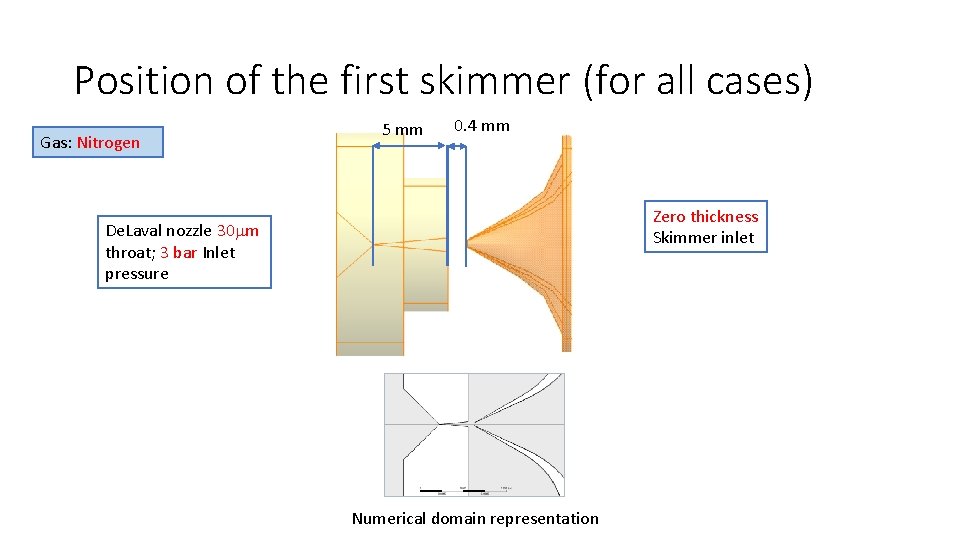
Position of the first skimmer (for all cases) Gas: Nitrogen 5 mm 0. 4 mm Zero thickness Skimmer inlet De. Laval nozzle 30 mm throat; 3 bar Inlet pressure Numerical domain representation
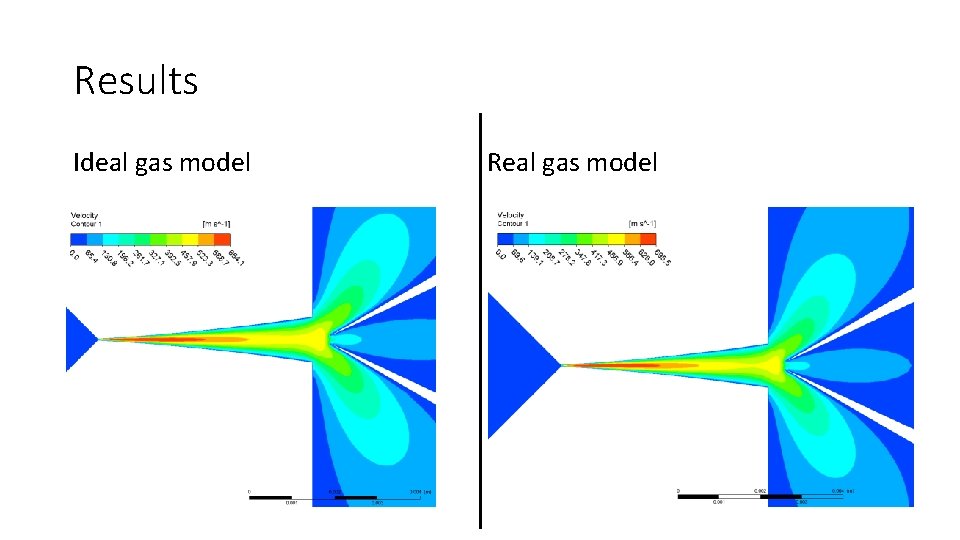
Results Ideal gas model Real gas model
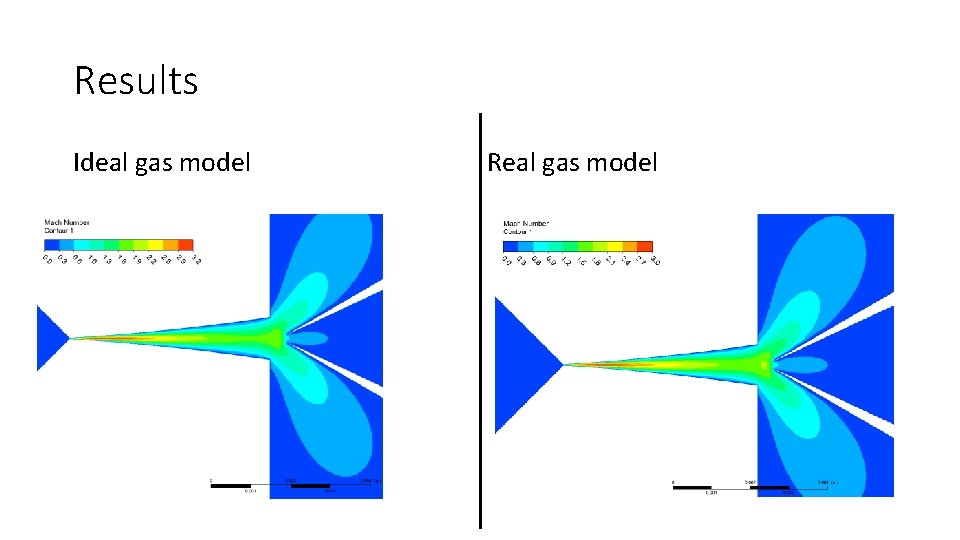
Results Ideal gas model Real gas model
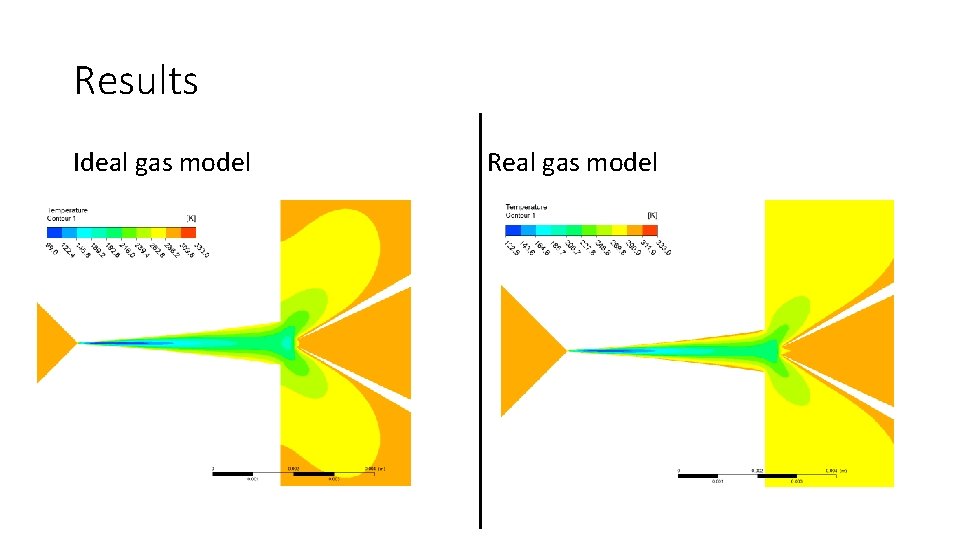
Results Ideal gas model Real gas model
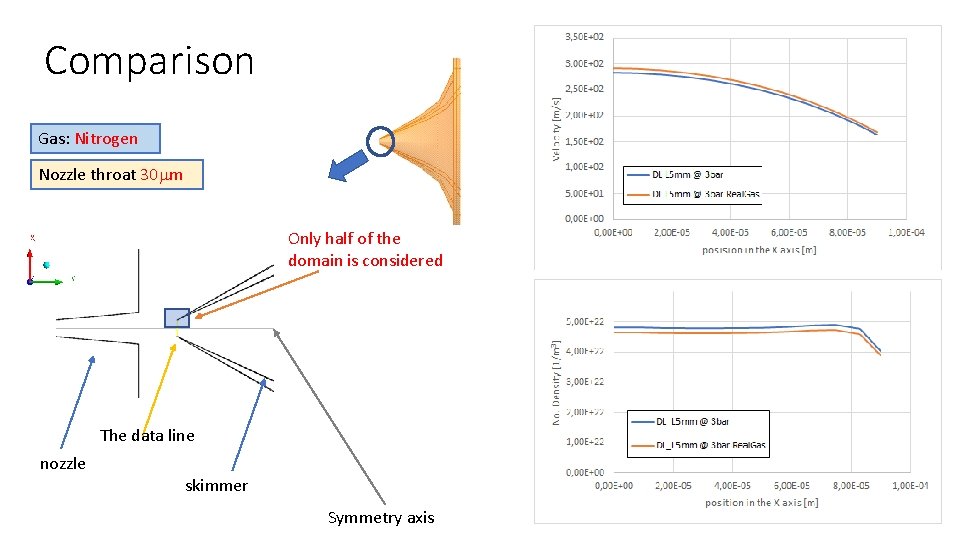
Comparison Gas: Nitrogen Nozzle throat 30 mm Only half of the domain is considered The data line nozzle skimmer Symmetry axis
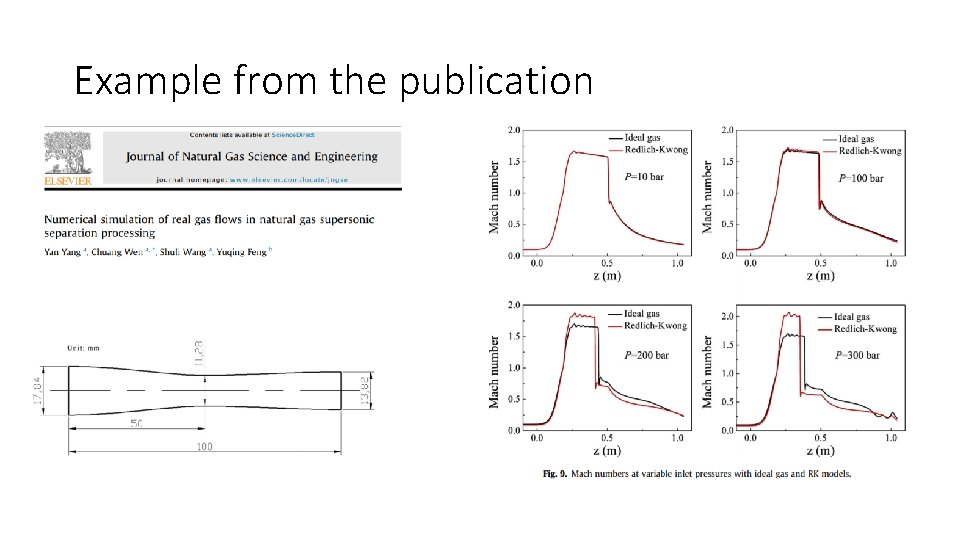
Example from the publication

Conclusions • There is no significant difference between real gas and ideal gas model for HP simulations • A difference of the numerical model on the border between HP and LP side is still unknown.
- Slides: 13