The Ideal Gas 1 Ideal gas equation of

- Slides: 21

The Ideal Gas 1

Ideal gas equation of state n n n Property tables provide very accurate information about the properties. It is desirable to have simple relations among the properties that are sufficiently general and accurate. Any equation that relates P-v-T are called Equation of state. Property relations that involve other properties of a substance at equilibrium states are also referred to as equations of state. The simplest and best-known equation of state for substances in gas phase is the ideal-gas equation of state. 2

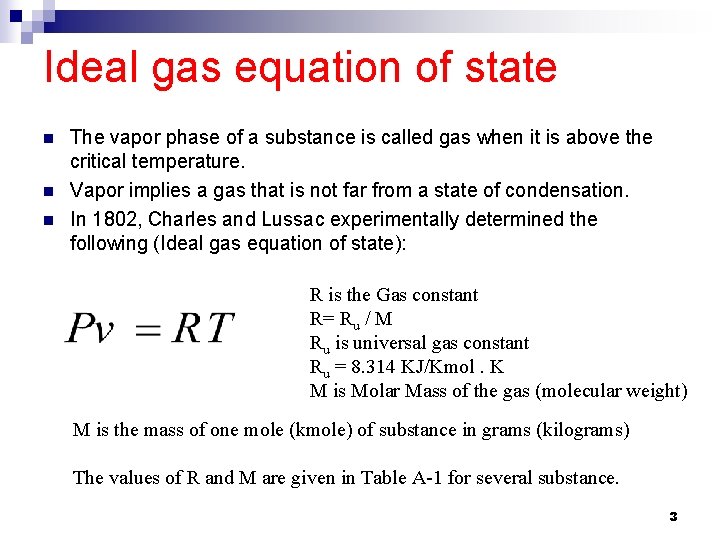
Ideal gas equation of state n n n The vapor phase of a substance is called gas when it is above the critical temperature. Vapor implies a gas that is not far from a state of condensation. In 1802, Charles and Lussac experimentally determined the following (Ideal gas equation of state): R is the Gas constant R= Ru / M Ru is universal gas constant Ru = 8. 314 KJ/Kmol. K M is Molar Mass of the gas (molecular weight) M is the mass of one mole (kmole) of substance in grams (kilograms) The values of R and M are given in Table A-1 for several substance. 3

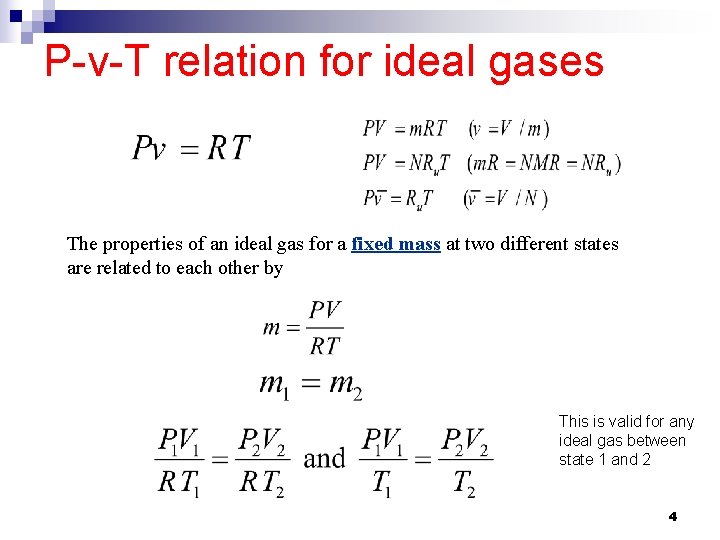
P-v-T relation for ideal gases The properties of an ideal gas for a fixed mass at two different states are related to each other by This is valid for any ideal gas between state 1 and 2 4

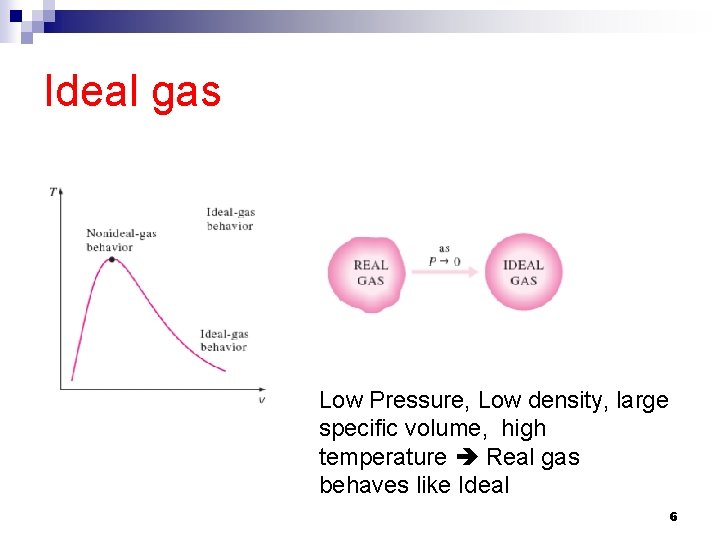
Ideal gas An ideal gas is an imaginary substance that obeys the P-v-T relation. The aforementioned relation approximates the behavior of real gas at low densities. At low pressure and high temperature, the density of a gas decreases, and the gas behaves like an ideal gas. In the range of practical interest, many familiar gases such as air, nitrogen, oxygen, hydrogen, helium, Aragon, neon, krypton, and even heavier gases such as carbon dioxide can be treated as ideal gases with negligible error (often less than 1 %). Dense gases such as water vapor in steam power plants and refrigerant vapor in refrigerators, however, should not be treated as ideal gases. Instead, the property tables should be used for these substances. 5

Ideal gas Low Pressure, Low density, large specific volume, high temperature Real gas behaves like Ideal 6

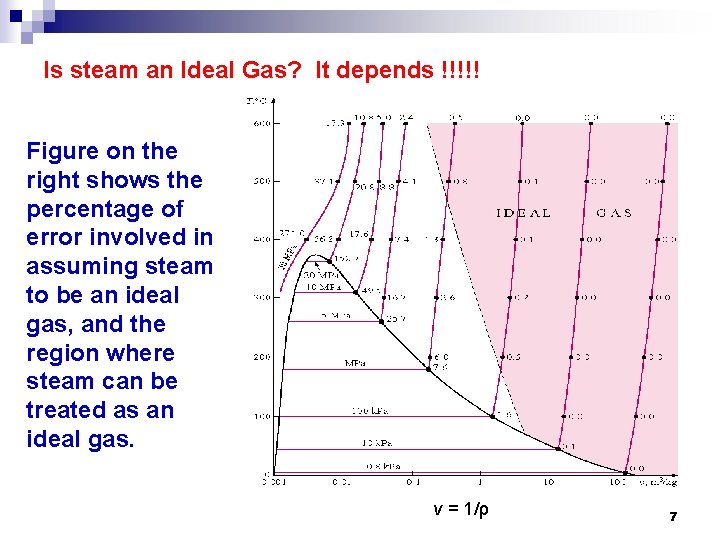
Is steam an Ideal Gas? It depends !!!!! Figure on the right shows the percentage of error involved in assuming steam to be an ideal gas, and the region where steam can be treated as an ideal gas. v = 1/ρ 7

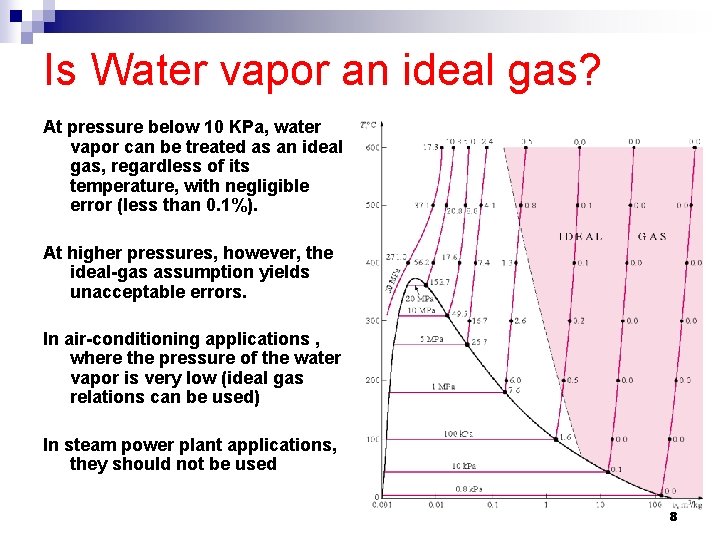
Is Water vapor an ideal gas? At pressure below 10 KPa, water vapor can be treated as an ideal gas, regardless of its temperature, with negligible error (less than 0. 1%). At higher pressures, however, the ideal-gas assumption yields unacceptable errors. In air-conditioning applications , where the pressure of the water vapor is very low (ideal gas relations can be used) In steam power plant applications, they should not be used 8

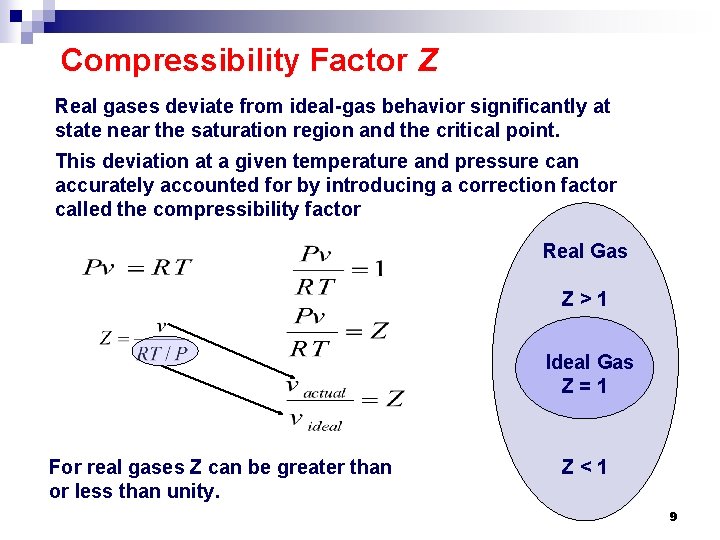
Compressibility Factor Z Real gases deviate from ideal-gas behavior significantly at state near the saturation region and the critical point. This deviation at a given temperature and pressure can accurately accounted for by introducing a correction factor called the compressibility factor Real Gas Z>1 Ideal Gas Z=1 For real gases Z can be greater than or less than unity. Z<1 9

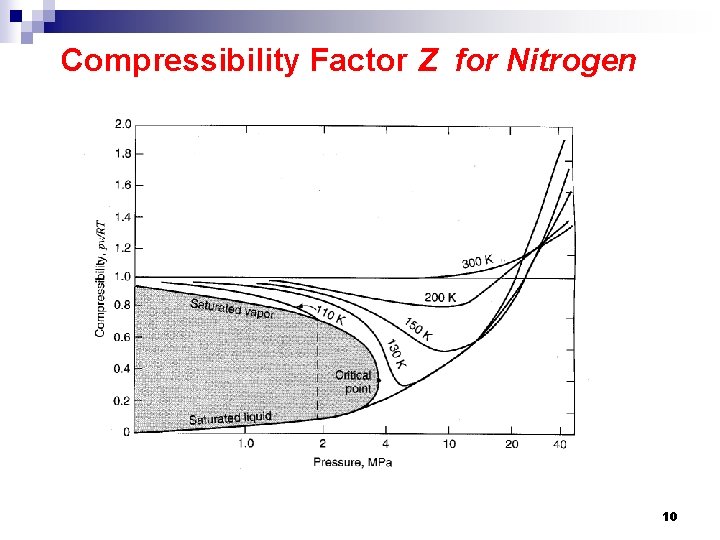
Compressibility Factor Z for Nitrogen 10

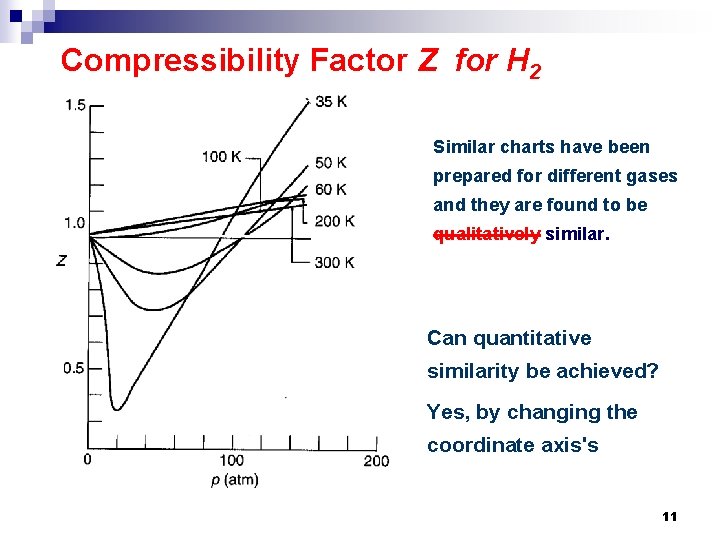
Compressibility Factor Z for H 2 Similar charts have been prepared for different gases and they are found to be qualitatively similar. Can quantitative similarity be achieved? Yes, by changing the coordinate axis's 11

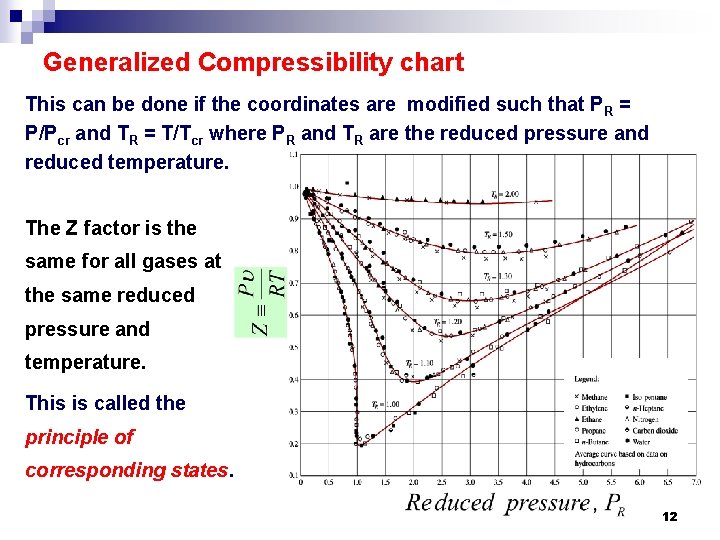
Generalized Compressibility chart This can be done if the coordinates are modified such that P R = P/Pcr and TR = T/Tcr where PR and TR are the reduced pressure and reduced temperature. The Z factor is the same for all gases at the same reduced pressure and temperature. This is called the principle of corresponding states. 12

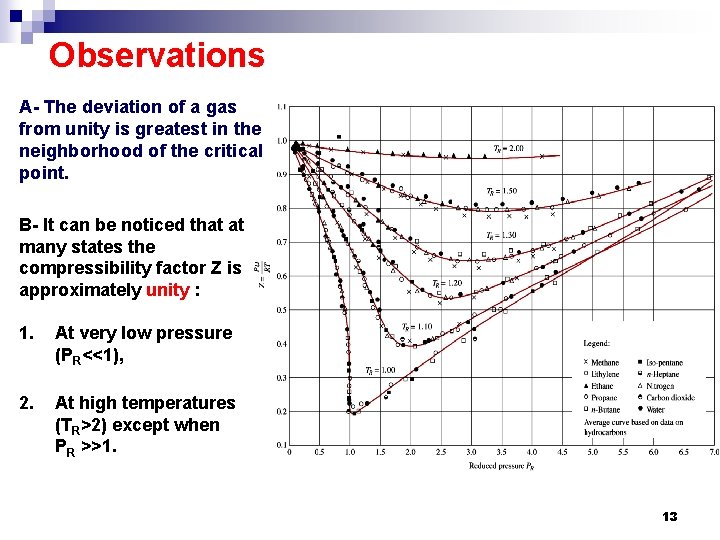
Observations A- The deviation of a gas from unity is greatest in the neighborhood of the critical point. B- It can be noticed that at many states the compressibility factor Z is approximately unity : 1. At very low pressure (PR<<1), 2. At high temperatures (TR>2) except when PR >>1. 13

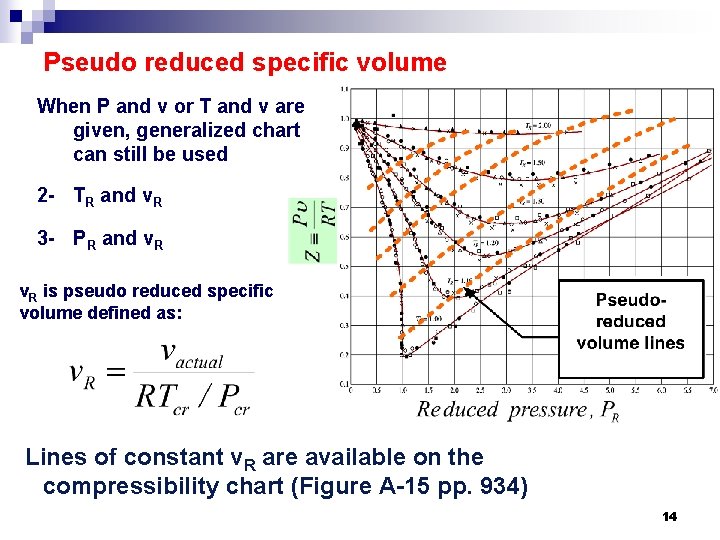
Pseudo reduced specific volume When P and v or T and v are given, generalized chart can still be used 2 - TR and v. R 3 - PR and v. R is pseudo reduced specific volume defined as: Lines of constant v. R are available on the compressibility chart (Figure A-15 pp. 934) 14

Example 3 -11 n Determine the specific volume of refiregerant-134 at 1 MPa and 50 C, using: Thermodynamic tables B. The ideal gas low C. The generalized compressibility chart. A. n Find the percentage error in the values obtained in B and C compared to the value obtained in part A. 15

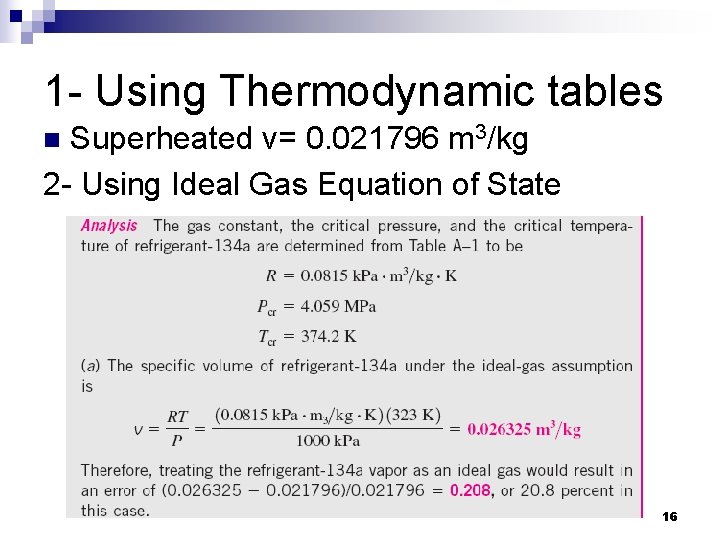
1 - Using Thermodynamic tables Superheated v= 0. 021796 m 3/kg 2 - Using Ideal Gas Equation of State n 16

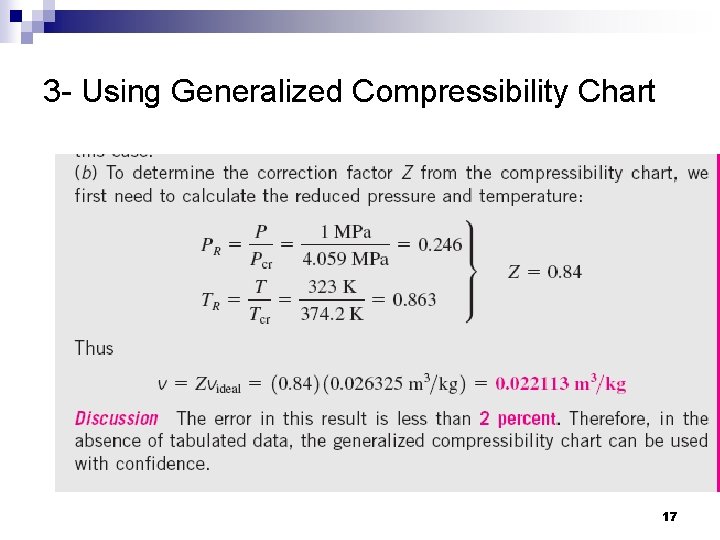
3 - Using Generalized Compressibility Chart 17

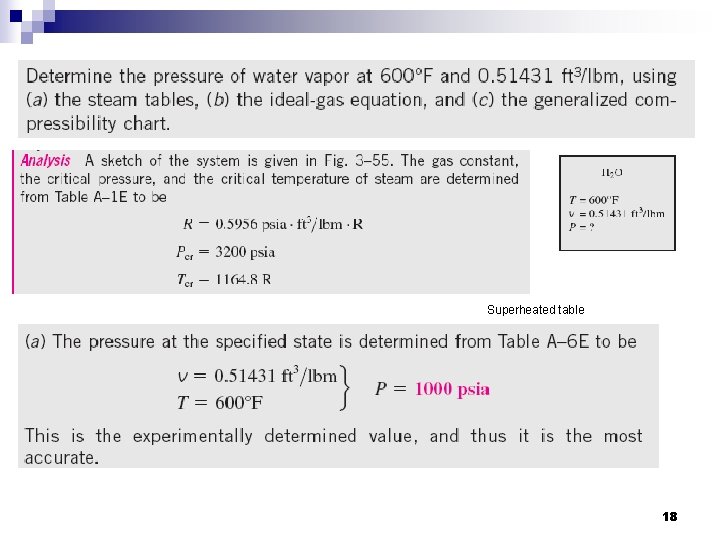
Superheated table 18

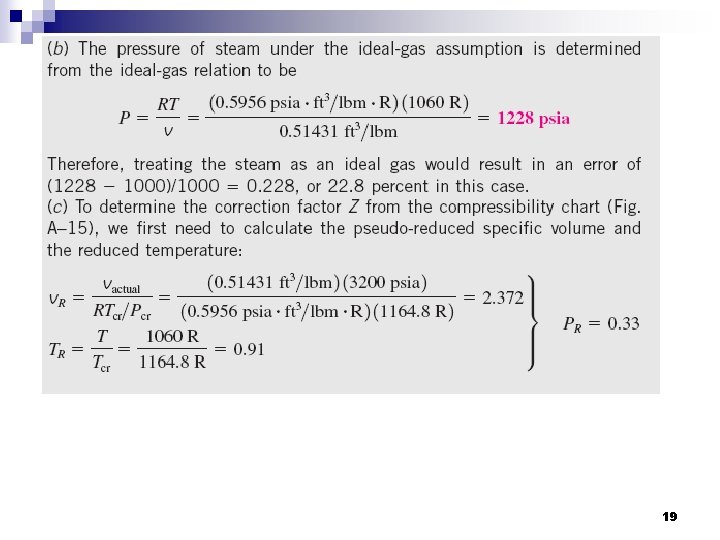
19

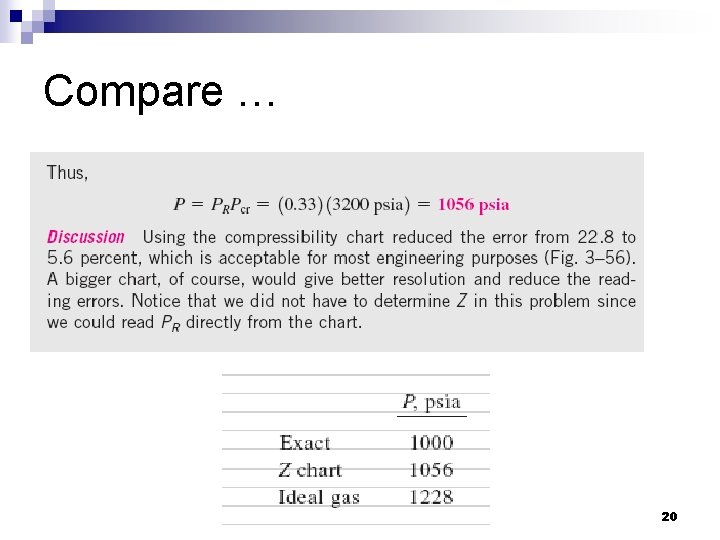
Compare … 20

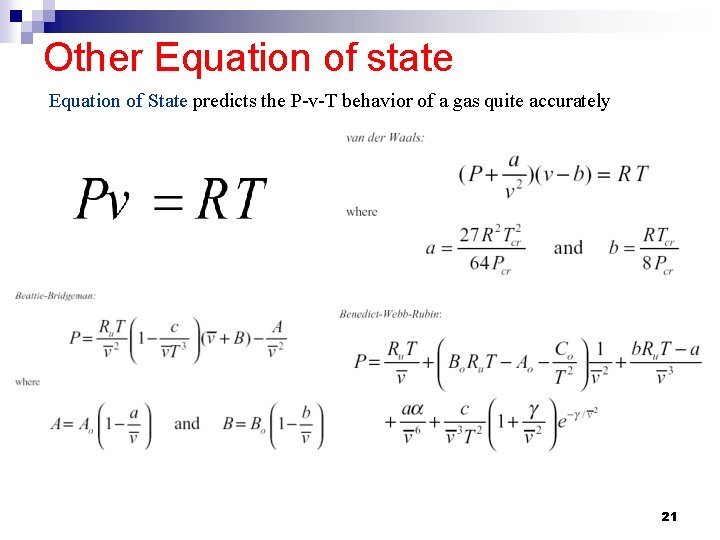
Other Equation of state Equation of State predicts the P-v-T behavior of a gas quite accurately 21