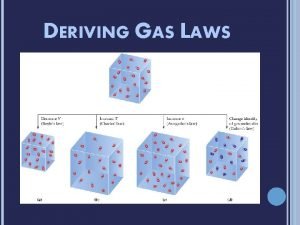
IDEAL GAS LAW Ideal Gas Law Derivation Recall












- Slides: 20

IDEAL GAS LAW

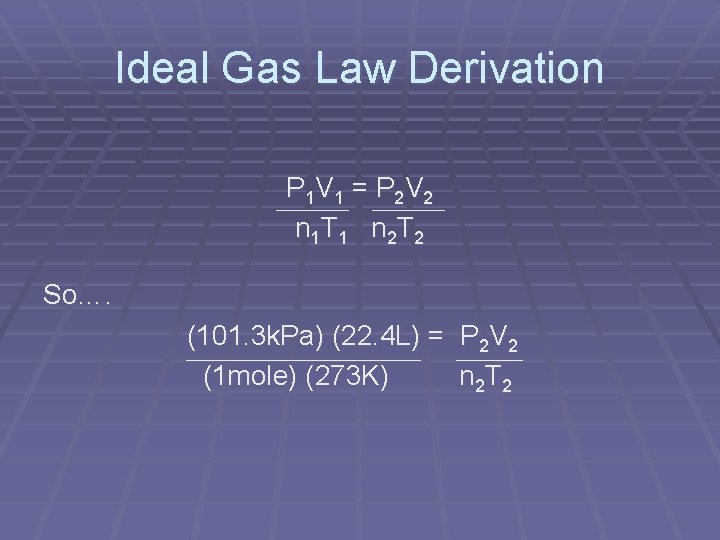
Ideal Gas Law Derivation § Recall that P 1 V 1 = P 2 V 2 n 1 T 1 n 2 T 2 § Also we learned that § At Oo. C, and 1 atm (101. 3 k. Pa) that 1 mole of gas occupies 22. 4 L (molar volume)

Ideal Gas Law Derivation § We can set one side of the equation under standard conditions ie. § n = 1 mole § V = 22. 4 L § P = 101. 3 k. Pa § T = 0 o. C

Ideal Gas Law Derivation P 1 V 1 = P 2 V 2 n 1 T 1 n 2 T 2 So…. (101. 3 k. Pa) (22. 4 L) = P 2 V 2 (1 mole) (273 K) n 2 T 2

Ideal Gas Law Derivation 8. 314 k. Pa x L = P V mol x K n. T Overall…. PV = n. RT 8. 314 k. Pa. L/mol K = R = Ideal Gas Law Constant

Ideal Gases l. Behave as described by the ideal gas equation; no real gas is actually ideal l. Within a few %, ideal gas equation describes most real gases at room temperature and pressures of 1 atm or less l. In real gases, particles attract each other reducing the pressure l. Real gases behave more like ideal gases as pressure approaches zero.

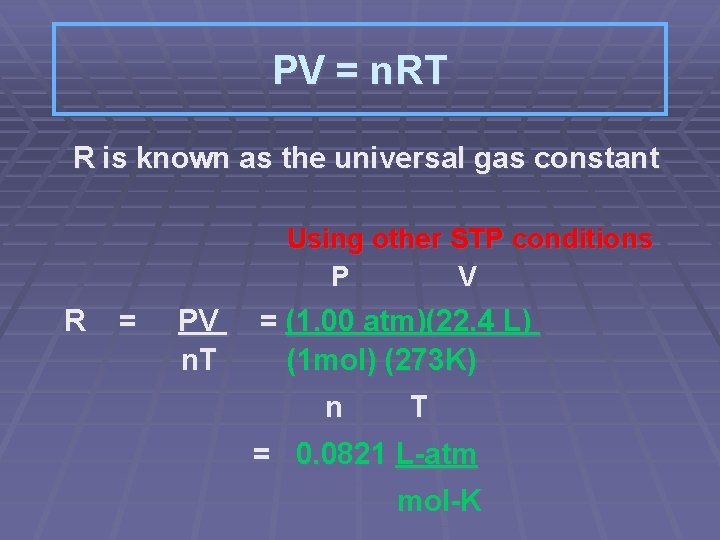
PV = n. RT R is known as the universal gas constant Using other STP conditions P V R = PV n. T = (1. 00 atm)(22. 4 L) (1 mol) (273 K) n T = 0. 0821 L-atm mol-K

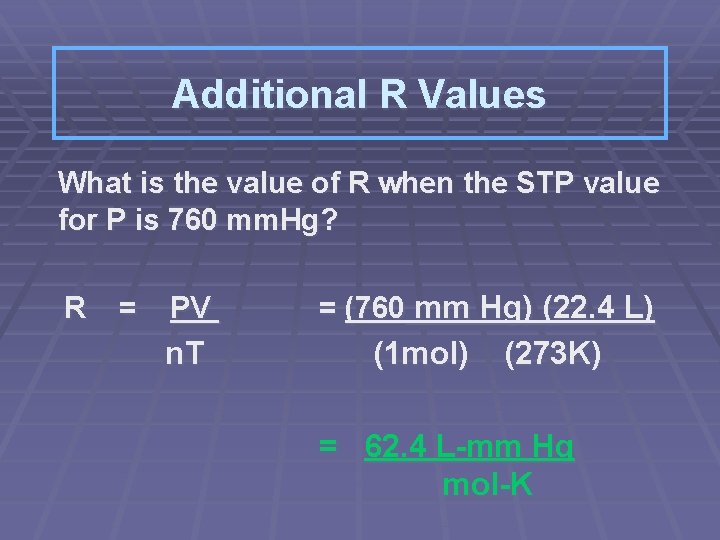
Additional R Values What is the value of R when the STP value for P is 760 mm. Hg? R = PV n. T = (760 mm Hg) (22. 4 L) (1 mol) (273 K) = 62. 4 L-mm Hg mol-K

Learning Check G 16 Dinitrogen monoxide (N 2 O), laughing gas, is used by dentists as an anesthetic. If 2. 86 mol of gas occupies a 20. 0 L tank at 23°C, what is the pressure (k. Pa) in the tank in the dentist office?

Solution G 16 Set up data for 3 of the 4 gas variables Adjust to match the units of R V = 20. 0 L T = 23°C + 273 296 K n = 2. 86 mol P = ? ?

Rearrange ideal gas law for unknown P P = n. RT V Substitute values of n, R, T and V and solve for P P = (2. 86 mol)(8. 314)(296 K) (20. 0 L) = 351. 91 k. Pa

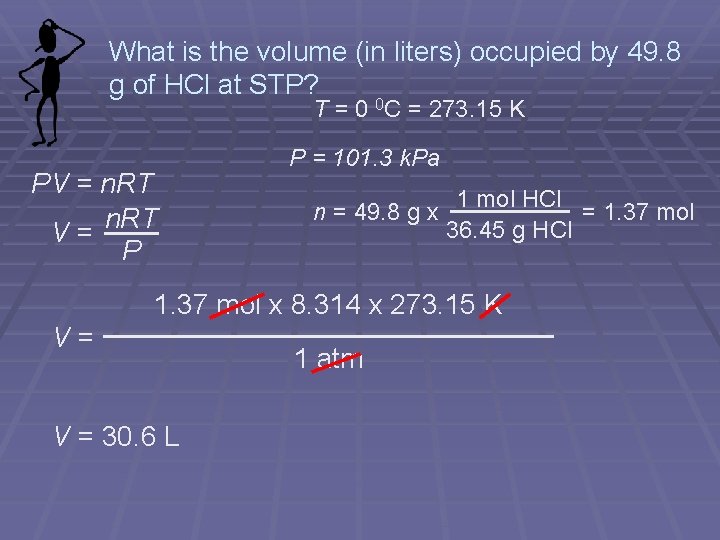
What is the volume (in liters) occupied by 49. 8 g of HCl at STP? T = 0 0 C = 273. 15 K PV = n. RT V= P P = 101. 3 k. Pa n = 49. 8 g x 1 mol HCl = 1. 37 mol 36. 45 g HCl 1. 37 mol x 8. 314 x 273. 15 K V= V = 30. 6 L 1 atm

Learning Check G 17 A 5. 0 L cylinder contains oxygen gas at 20. 0°C and 98 k. Pa. How many grams of oxygen are in the cylinder?

Solution G 17 Solve ideal gas equation for n (moles) n = PV RT = (98 k. Pa)(5. 0 L)(mol K) (62. 4 mm. Hg L)(293 K) = 0. 20 mol O 2 x 32. 0 g O 2 = 6. 4 g O 2 1 mol O 2

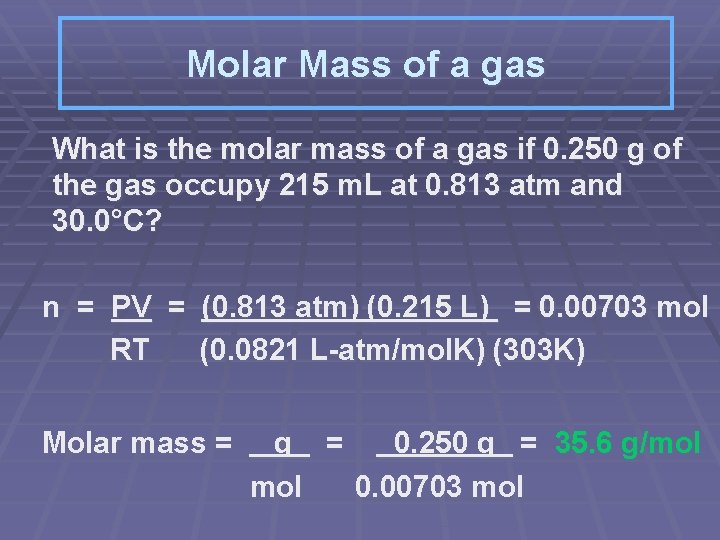
Molar Mass of a gas What is the molar mass of a gas if 0. 250 g of the gas occupy 215 m. L at 0. 813 atm and 30. 0°C? n = PV = (0. 813 atm) (0. 215 L) = 0. 00703 mol RT (0. 0821 L-atm/mol. K) (303 K) Molar mass = g = 0. 250 g = 35. 6 g/mol 0. 00703 mol

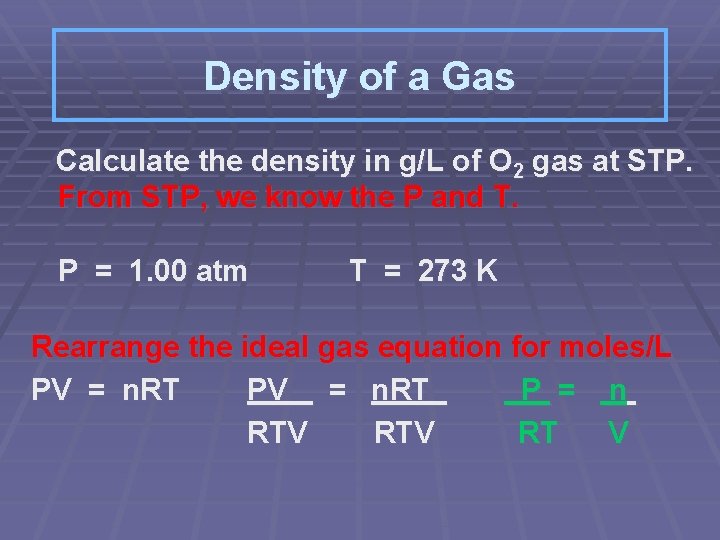
Density of a Gas Calculate the density in g/L of O 2 gas at STP. From STP, we know the P and T. P = 1. 00 atm T = 273 K Rearrange the ideal gas equation for moles/L PV = n. RT P = n RTV RT V

Substitute (1. 00 atm ) mol-K = (0. 0821 L-atm) (273 K) 0. 0446 mol O 2/L Change moles/L to g/L 0. 0446 mol O 2 1 L x 32. 0 g O 2 1 mol O 2 = 1. 43 g/L Therefore the density of O 2 gas at STP is 1. 43 grams per liter

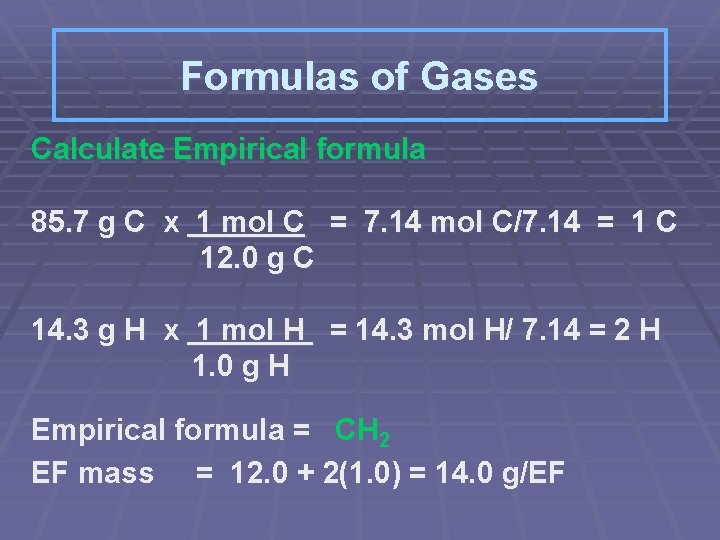
Formulas of Gases A gas has a % composition by mass of 85. 7% carbon and 14. 3% hydrogen. At STP the density of the gas is 2. 50 g/L. What is the molecular formula of the gas?

Formulas of Gases Calculate Empirical formula 85. 7 g C x 1 mol C = 7. 14 mol C/7. 14 = 1 C 12. 0 g C 14. 3 g H x 1 mol H = 14. 3 mol H/ 7. 14 = 2 H 1. 0 g H Empirical formula = CH 2 EF mass = 12. 0 + 2(1. 0) = 14. 0 g/EF

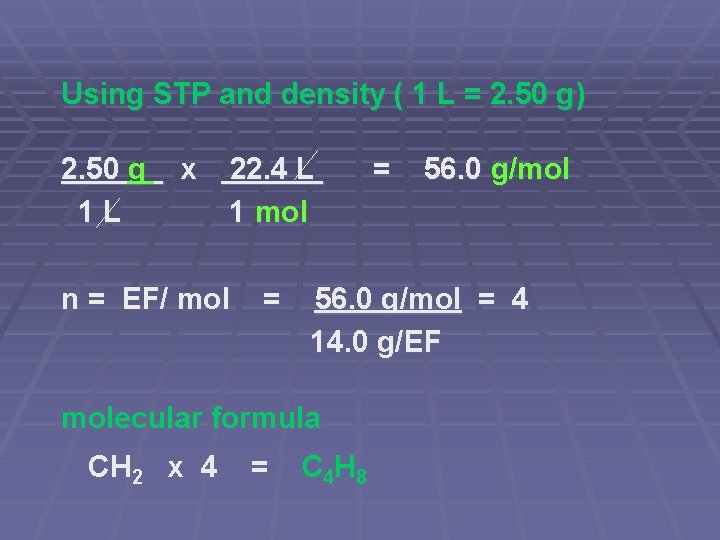
Using STP and density ( 1 L = 2. 50 g) 2. 50 g 1 L x 22. 4 L 1 mol n = EF/ mol = = 56. 0 g/mol = 4 14. 0 g/EF molecular formula CH 2 x 4 = C 4 H 8
 Ideal gas law derivation
Ideal gas law derivation Leftmost derivation and rightmost derivation
Leftmost derivation and rightmost derivation Diode equation derivation
Diode equation derivation Ideal gas vs perfect gas
Ideal gas vs perfect gas An ideal gas is an imaginary gas
An ideal gas is an imaginary gas Gas law
Gas law Ideal gas vs perfect gas
Ideal gas vs perfect gas Difference between ideal gas and real gas
Difference between ideal gas and real gas Lambert beer law
Lambert beer law What is a critical angle
What is a critical angle Derivation of darcy's law
Derivation of darcy's law Snell's law derivation class 10
Snell's law derivation class 10 Boyle's law formula for p1
Boyle's law formula for p1 Darcy velocity vs actual velocity
Darcy velocity vs actual velocity The flux of the electric field (24 n/c)
The flux of the electric field (24 n/c) Definition of colligative property
Definition of colligative property Faraday's law of induction derivation
Faraday's law of induction derivation Dalton's law derivation
Dalton's law derivation Newtons third law of thermodynamics
Newtons third law of thermodynamics Derivation of ohm's law
Derivation of ohm's law закон био савара лапласа
закон био савара лапласа