Gas Stoichiometry using The Ideal Gas Law Ideal



- Slides: 11

Gas Stoichiometry using The Ideal Gas Law

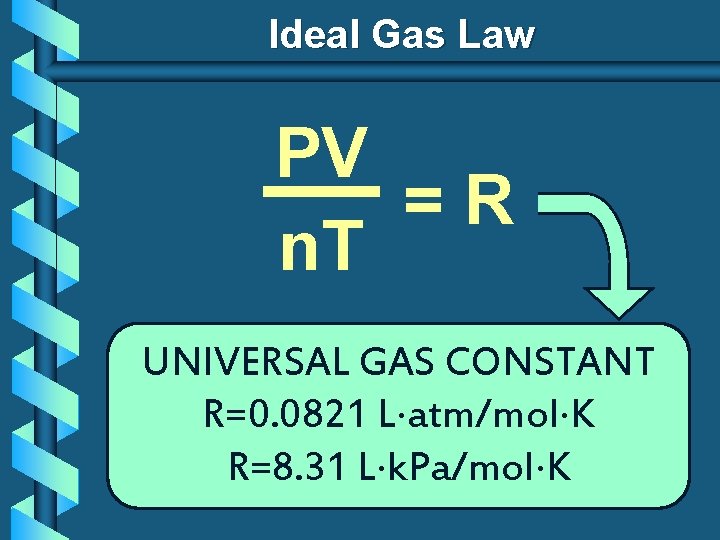
Ideal Gas Law V PV k =R n n. T T UNIVERSAL GAS CONSTANT R=0. 0821 L atm/mol K R=8. 31 L k. Pa/mol K

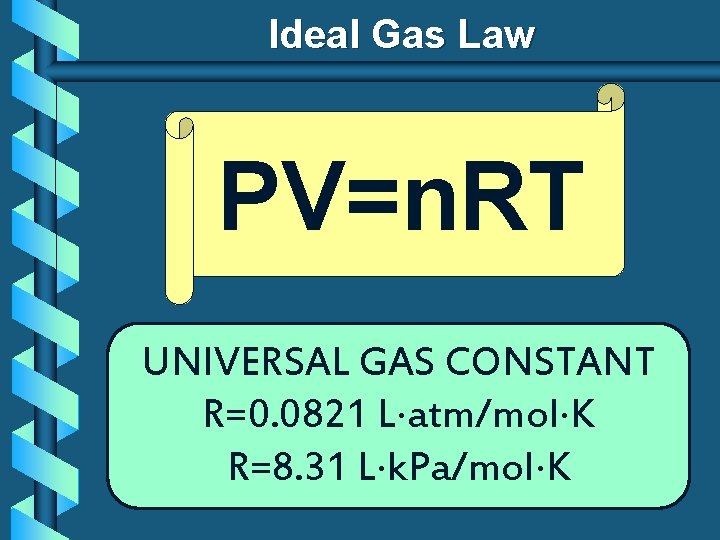
Ideal Gas Law PV=n. RT UNIVERSAL GAS CONSTANT R=0. 0821 L atm/mol K R=8. 31 L k. Pa/mol K

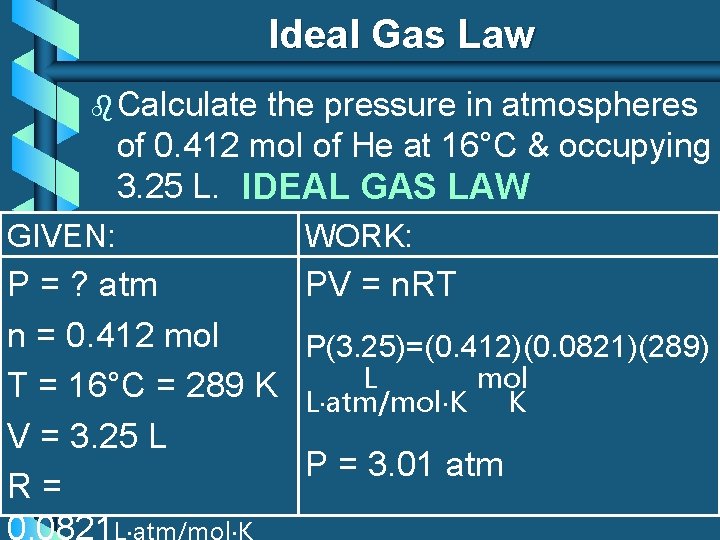
Ideal Gas Law b Calculate the pressure in atmospheres of 0. 412 mol of He at 16°C & occupying 3. 25 L. IDEAL GAS LAW GIVEN: WORK: P = ? atm PV = n. RT n = 0. 412 mol P(3. 25)=(0. 412)(0. 0821)(289) L mol T = 16°C = 289 K L atm/mol K K V = 3. 25 L P = 3. 01 atm R= 0. 0821 L atm/mol K

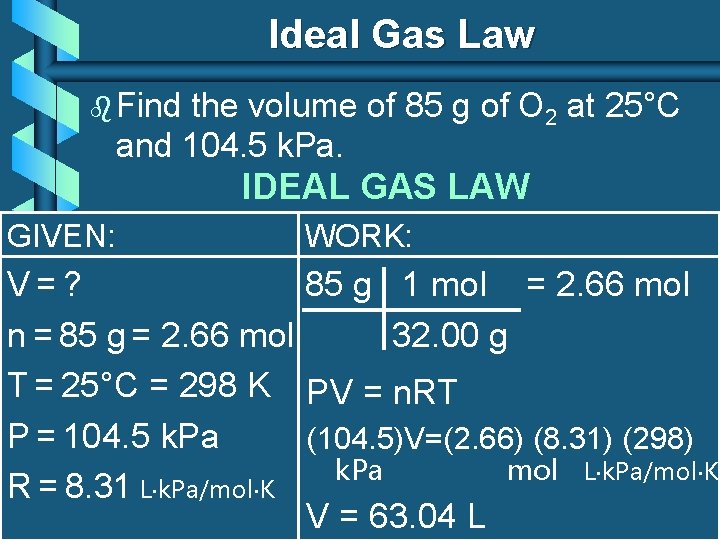
Ideal Gas Law b Find the volume of 85 g of O 2 at 25°C and 104. 5 k. Pa. IDEAL GAS LAW GIVEN: WORK: V=? 85 g 1 mol = 2. 66 mol n = 85 g = 2. 66 mol 32. 00 g T = 25°C = 298 K PV = n. RT P = 104. 5 k. Pa (104. 5)V=(2. 66) (8. 31) (298) k. Pa mol L k. Pa/mol K R = 8. 31 L k. Pa/mol K V = 63. 04 L

Gas Stoichiometry use at non-STP Conditions

Gas Stoichiometry b Moles Liters of a Gas • STP Ø use 22. 4 L/mol (Avogadro’s Law) • Non-STP Ø use ideal gas law b Non-STP Problems • Given liters of gas? Ø start with ideal gas law • Looking for liters of gas? Ø start with stoichiometry conversion

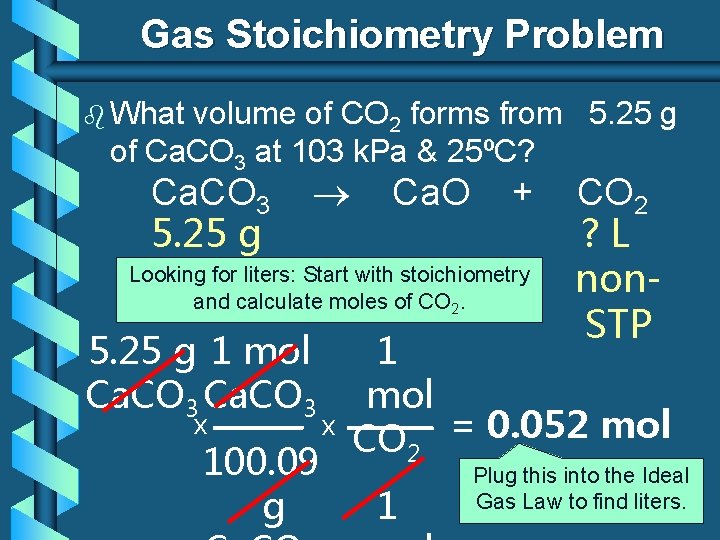
Gas Stoichiometry Problem b What volume of CO 2 forms from 5. 25 g of Ca. CO 3 at 103 k. Pa & 25ºC? Ca. CO 3 5. 25 g Ca. O + Looking for liters: Start with stoichiometry and calculate moles of CO 2. 5. 25 g 1 mol Ca. CO 3 CO 2 ? L non. STP 1 mol x x = 0. 052 mol CO 2 100. 09 CO Plug this 2 into the Ideal Gas Law to find liters. g 1

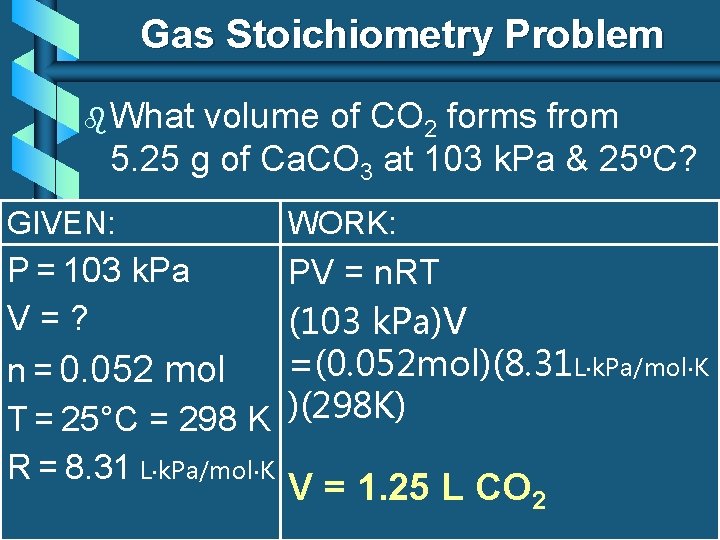
Gas Stoichiometry Problem b What volume of CO 2 forms from 5. 25 g of Ca. CO 3 at 103 k. Pa & 25ºC? GIVEN: WORK: P = 103 k. Pa V=? n = 0. 052 mol T = 25°C = 298 K R = 8. 31 L k. Pa/mol K PV = n. RT (103 k. Pa)V =(0. 052 mol)(8. 31 L k. Pa/mol K )(298 K) V = 1. 25 L CO 2

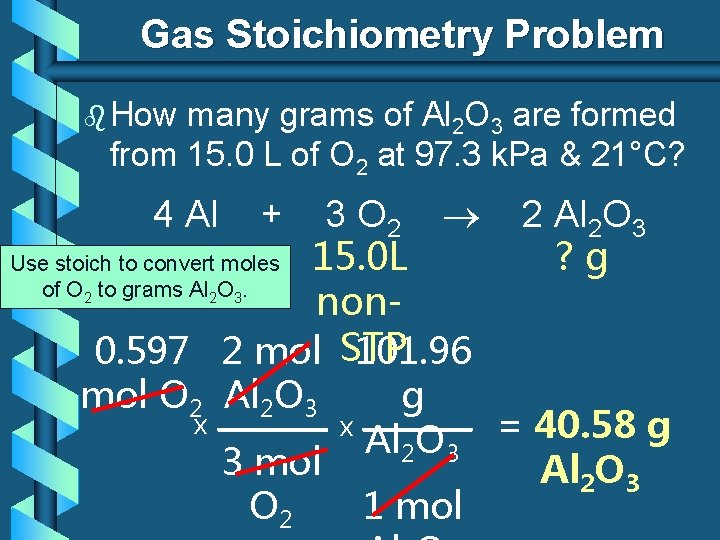
Gas Stoichiometry Problem b How many grams of Al 2 O 3 are formed from 15. 0 L of O 2 at 97. 3 k. Pa & 21°C? 4 Al + GIVEN: P = 97. 3 k. Pa V = 15. 0 L n=? T = 21°C = 294 K R = 8. 31 L k. Pa/mol K 3 O 2 15. 0 L non. STP WORK: 2 Al 2 O 3 ? g Given liters: Start with Ideal Gas Law and calculate moles of O 2. PV = n. RT (97. 3 k. Pa) (15. 0 L) = n (8. 31 L k. Pa/mol K) NEXT (294 K)

Gas Stoichiometry Problem b How many grams of Al 2 O 3 are formed from 15. 0 L of O 2 at 97. 3 k. Pa & 21°C? 3 O 2 2 Al 2 O 3 ? g Use stoich to convert moles 15. 0 L of O to grams Al O. non 0. 597 2 mol STP 101. 96 mol O 2 Al 2 O 3 g x x = 40. 58 g Al 2 O 3 3 mol Al 2 O 3 O 2 1 mol 4 Al 2 2 + 3