Ideal Gas Law Chapter 14 3 Ideal Gas














- Slides: 21

Ideal Gas Law Chapter 14. 3

Ideal Gas Law • The ideal gas law combines: – pressure – temperature – volume – # of particles (amount)

Increasing Amount of Particles • If the amount of gas particles increases: – the pressure will increase OR – the volume will increase

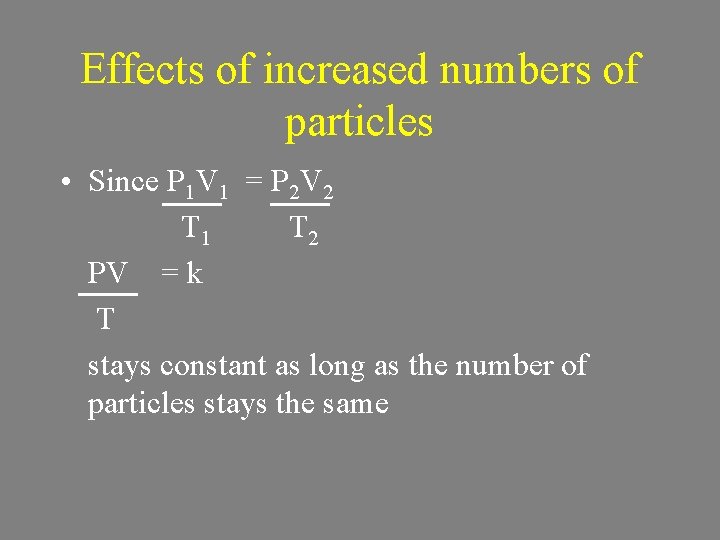
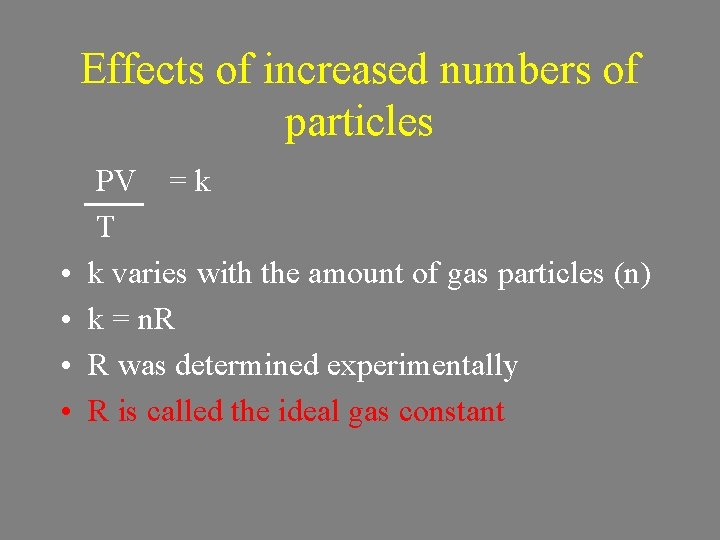
Effects of increased numbers of particles • Since P 1 V 1 = P 2 V 2 T 1 T 2 PV = k T stays constant as long as the number of particles stays the same

Effects of increased numbers of particles • • PV = k T k varies with the amount of gas particles (n) k = n. R R was determined experimentally R is called the ideal gas constant

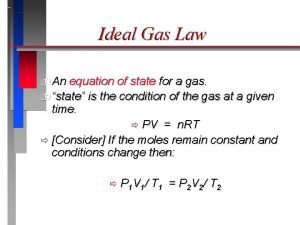
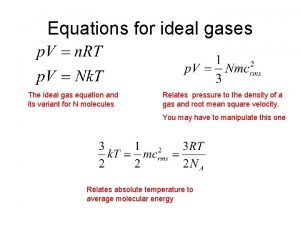
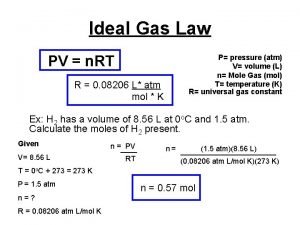
Vocabulary Word • ideal gas law: describes the physical behavior of an ideal gas in terms of the temperature, volume and pressure and the number of moles of a gas that are present • PV = n. RT

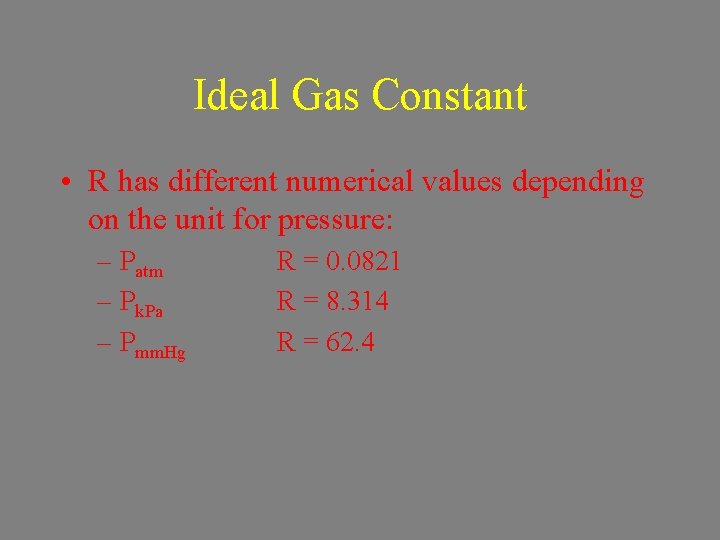
Ideal Gas Constant • R has different numerical values depending on the unit for pressure: – Patm – Pk. Pa – Pmm. Hg R = 0. 0821 R = 8. 314 R = 62. 4

Units for the Ideal Gas Law • Volume (liters) • Temp (Kelvin) • n (moles)

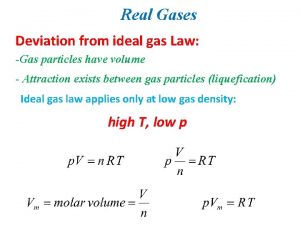
Properties of Ideal Gases • the gas particles have no intermolecular forces of attraction or repulsion • in the real world gas particles DO have a small but measurable volume

Real Gases • most gases behave like ideal gases at many temperatures and pressures • we can use the ideal gas law to get a very close approximation of experimentally verified values

Real Gases • at extremely high pressures or low temperature intermolecular forces become important • this allows gases to liquify

Intermolecular Forces • size and geometry (shape) can increase the intermolecular forces of attraction, • values calculated with the ideal gas law will be off – polar gases (water vapor) – larger gases (butane)

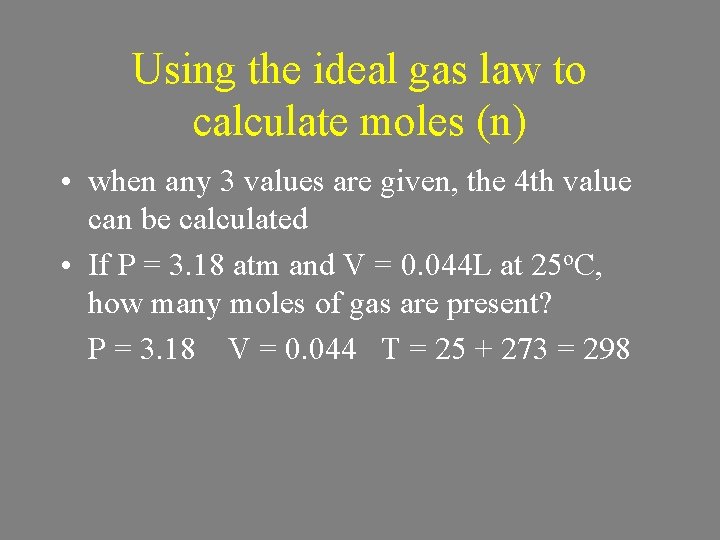
Using the ideal gas law to calculate moles (n) • when any 3 values are given, the 4 th value can be calculated • If P = 3. 18 atm and V = 0. 044 L at 25 o. C, how many moles of gas are present? P = 3. 18 V = 0. 044 T = 25 + 273 = 298

Using the ideal gas law to calculate moles (n) P = 3. 18 V = 0. 044 T = 25 + 273 = 298 PV = n. RT (3. 18) (0. 044) = n (0. 0821) (298)

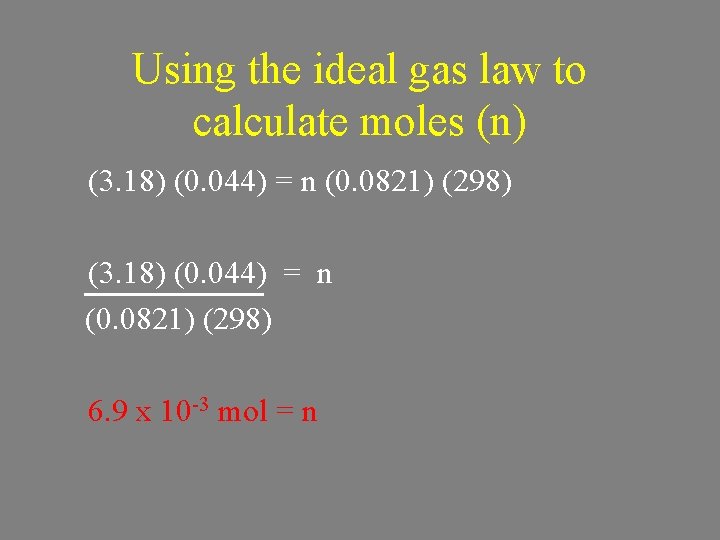
Using the ideal gas law to calculate moles (n) (3. 18) (0. 044) = n (0. 0821) (298) 6. 9 x 10 -3 mol = n

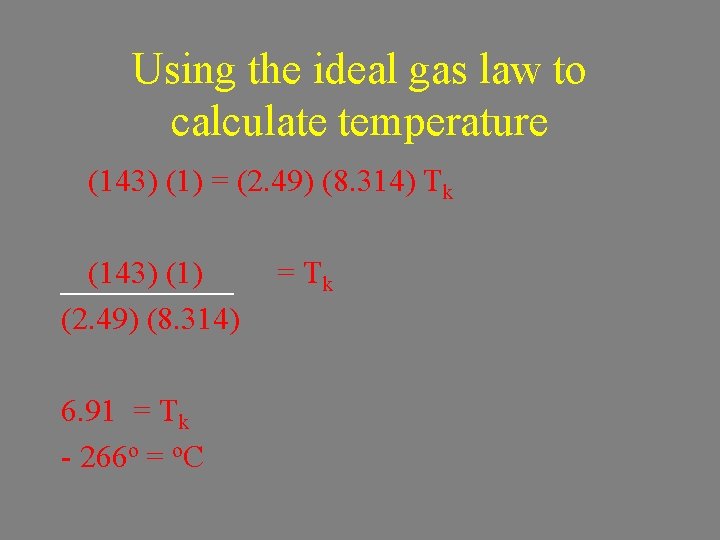
Using the ideal gas law to calculate temperature n = 2. 49 mol V = 1 L P = 143 k. Pa T=? (143) (1) = (2. 49) (8. 314) Tk

Using the ideal gas law to calculate temperature (143) (1) = (2. 49) (8. 314) Tk (143) (1) (2. 49) (8. 314) 6. 91 = Tk - 266 o = o. C = Tk

Using the ideal gas law to calculate volume • n = 0. 323 mol • T= 265 K • P = 0. 900 atm (0. 900) V = (0. 323) (0. 0821) (265) (0. 900) = 7. 821 L


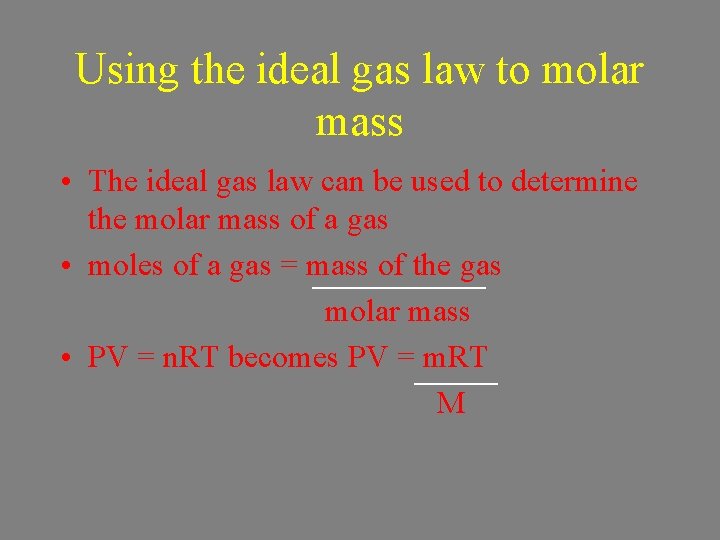
Using the ideal gas law to molar mass • The ideal gas law can be used to determine the molar mass of a gas • moles of a gas = mass of the gas molar mass • PV = n. RT becomes PV = m. RT M

PV = m. RT M solve for M, and M = m. RT PV
 Pseudo reduced specific volume
Pseudo reduced specific volume An ideal gas is an imaginary gas
An ideal gas is an imaginary gas Differences between ideal gas and real gas
Differences between ideal gas and real gas Computational fluid dynamics
Computational fluid dynamics Difference between ideal gas and real gas
Difference between ideal gas and real gas Unit of pressure
Unit of pressure Gas law formulas
Gas law formulas Ideal gas law examples
Ideal gas law examples Which equation agrees with the ideal gas law?
Which equation agrees with the ideal gas law? Deviation from ideal gas
Deviation from ideal gas Ideal gas law example
Ideal gas law example Deviations from the ideal gas law
Deviations from the ideal gas law Graph of ideal gas equation
Graph of ideal gas equation Ideal gas law density
Ideal gas law density State ideal gas equation
State ideal gas equation Pv=nrt units
Pv=nrt units Ideal gas equation
Ideal gas equation Ideal gas law formula
Ideal gas law formula How to find density in ideal gas law
How to find density in ideal gas law Ideal gas law to find density
Ideal gas law to find density Pzmore
Pzmore Ideal gas law find n
Ideal gas law find n