Lecture 16 Deviations from the Ideal Gas Law




- Slides: 15

Lecture #16 Deviations from the Ideal Gas Law and Chemistry in the Atmosphere Chemistry 142 B Autumn Quarter, 2004 J. B. Callis, Instructor


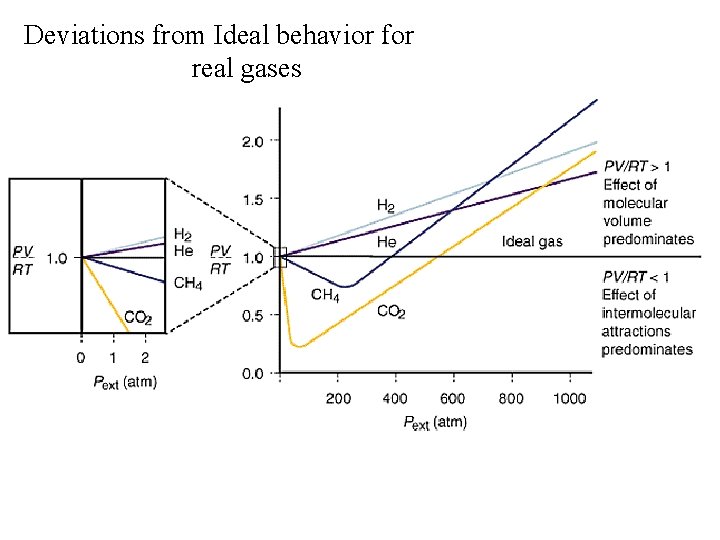
Deviations from Ideal behavior for real gases

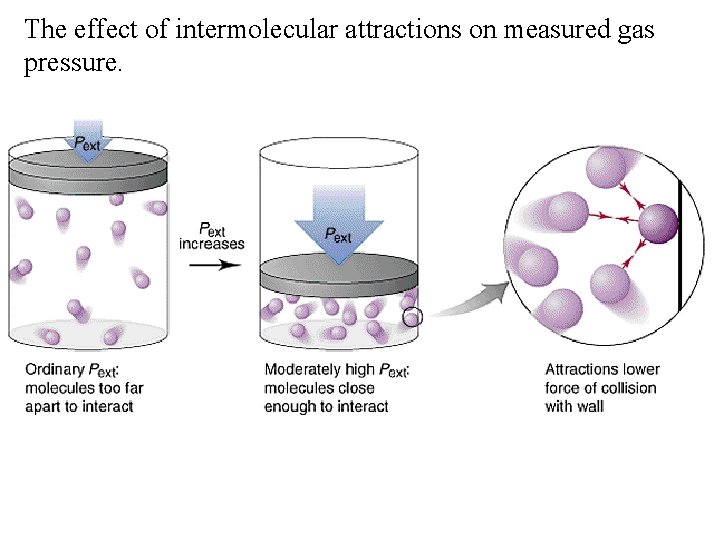
The effect of intermolecular attractions on measured gas pressure.

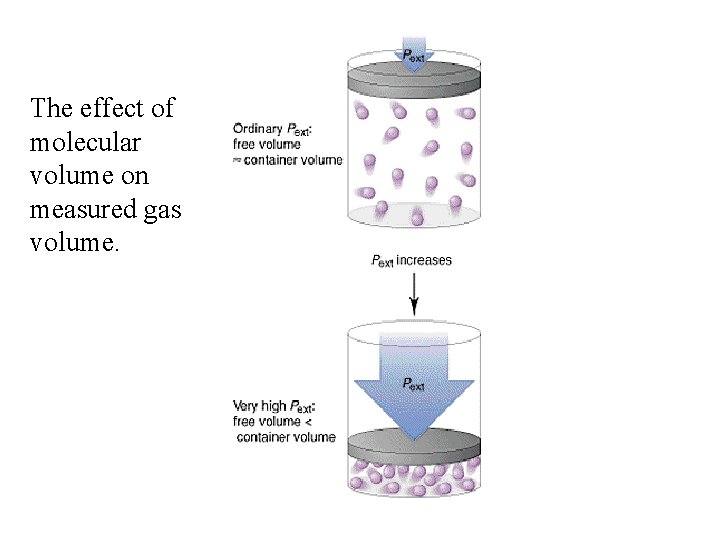
The effect of molecular volume on measured gas volume.

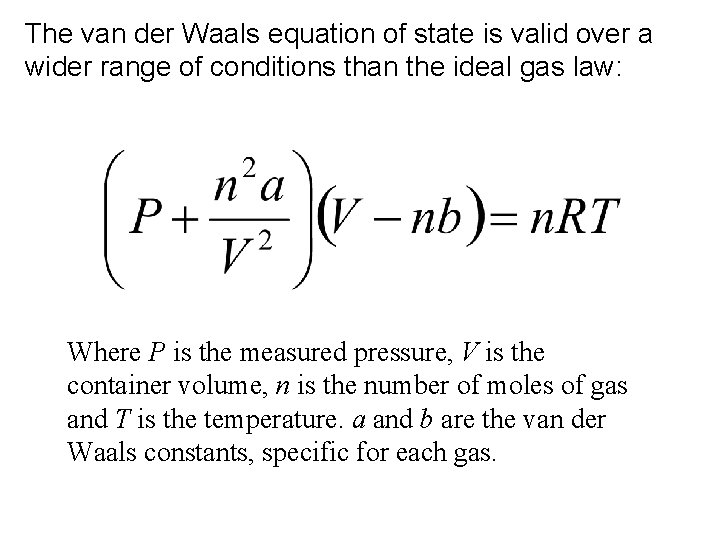
The van der Waals equation of state is valid over a wider range of conditions than the ideal gas law: Where P is the measured pressure, V is the container volume, n is the number of moles of gas and T is the temperature. a and b are the van der Waals constants, specific for each gas.

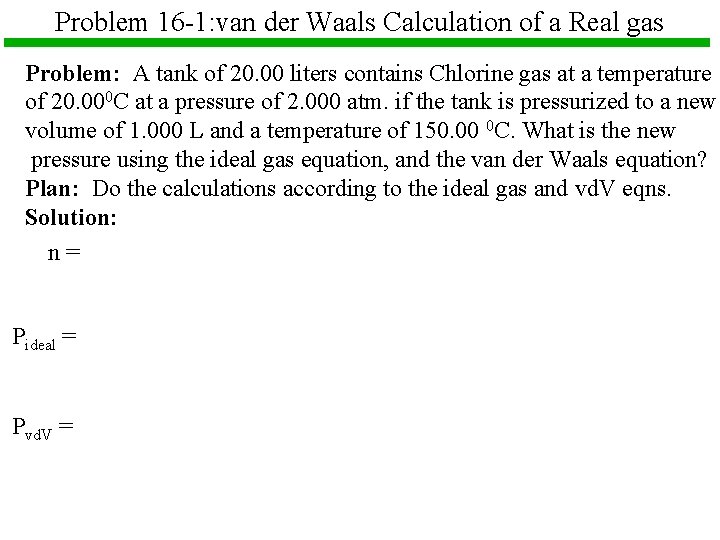
Problem 16 -1: van der Waals Calculation of a Real gas Problem: A tank of 20. 00 liters contains Chlorine gas at a temperature of 20. 000 C at a pressure of 2. 000 atm. if the tank is pressurized to a new volume of 1. 000 L and a temperature of 150. 00 0 C. What is the new pressure using the ideal gas equation, and the van der Waals equation? Plan: Do the calculations according to the ideal gas and vd. V eqns. Solution: n= Pideal = Pvd. V =

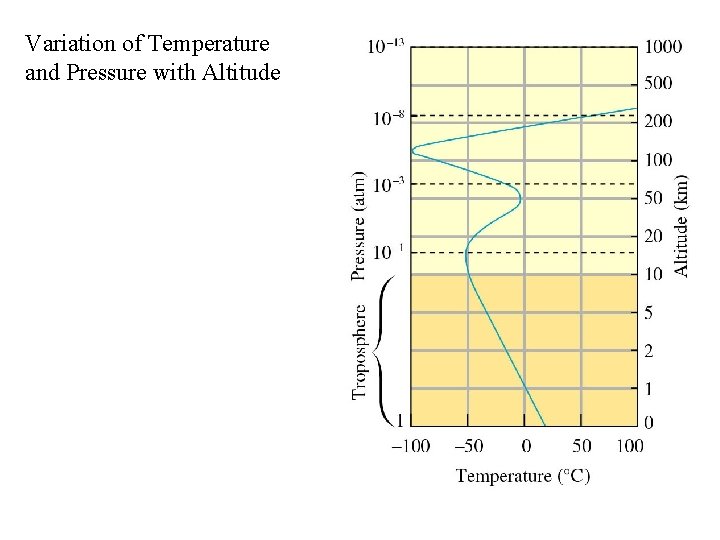
Variation of Temperature and Pressure with Altitude

Sources of Air Pollution • Transportation • Production of Electricity

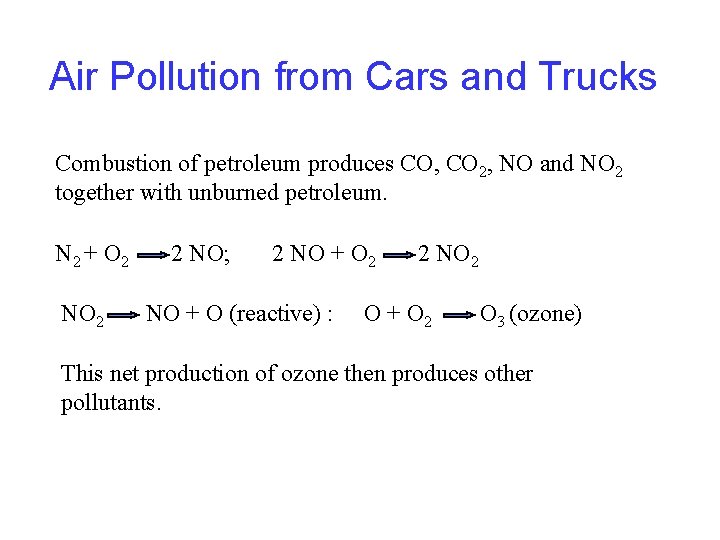
Air Pollution from Cars and Trucks Combustion of petroleum produces CO, CO 2, NO and NO 2 together with unburned petroleum. N 2 + O 2 NO 2 2 NO; 2 NO + O (reactive) : 2 NO 2 O + O 2 O 3 (ozone) This net production of ozone then produces other pollutants.

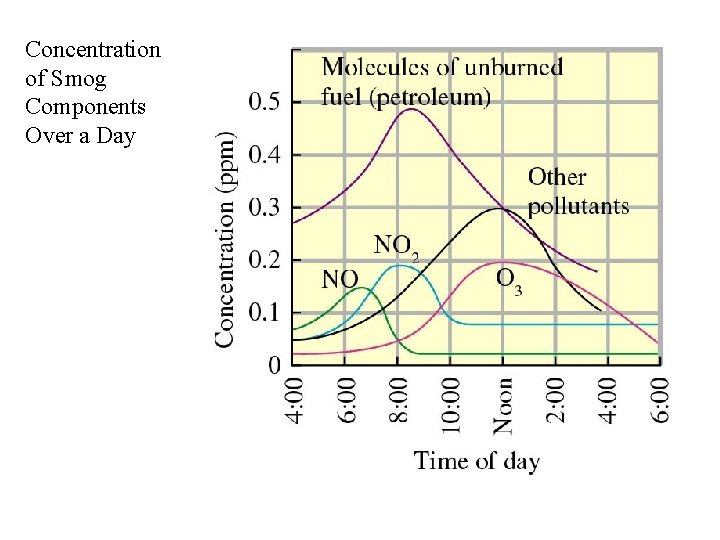
Concentration of Smog Components Over a Day

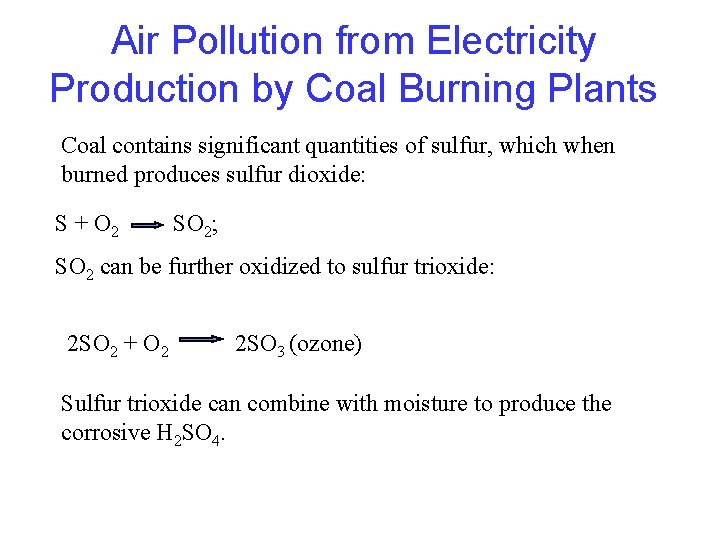
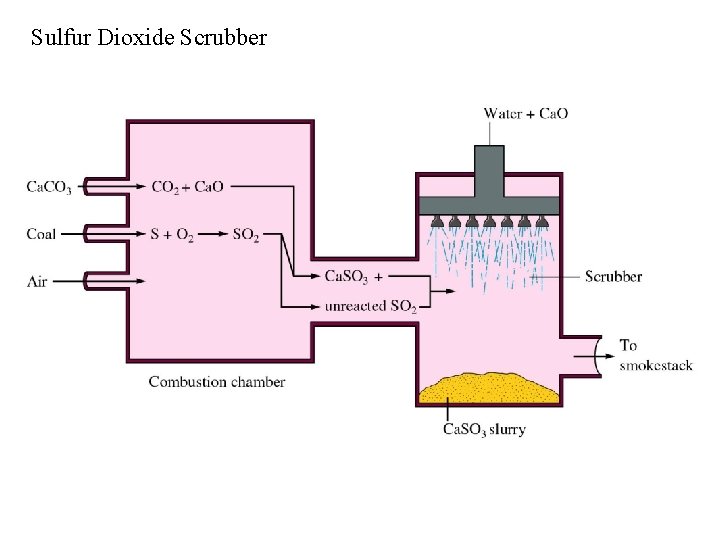
Air Pollution from Electricity Production by Coal Burning Plants Coal contains significant quantities of sulfur, which when burned produces sulfur dioxide: S + O 2 SO 2; SO 2 can be further oxidized to sulfur trioxide: 2 SO 2 + O 2 2 SO 3 (ozone) Sulfur trioxide can combine with moisture to produce the corrosive H 2 SO 4.

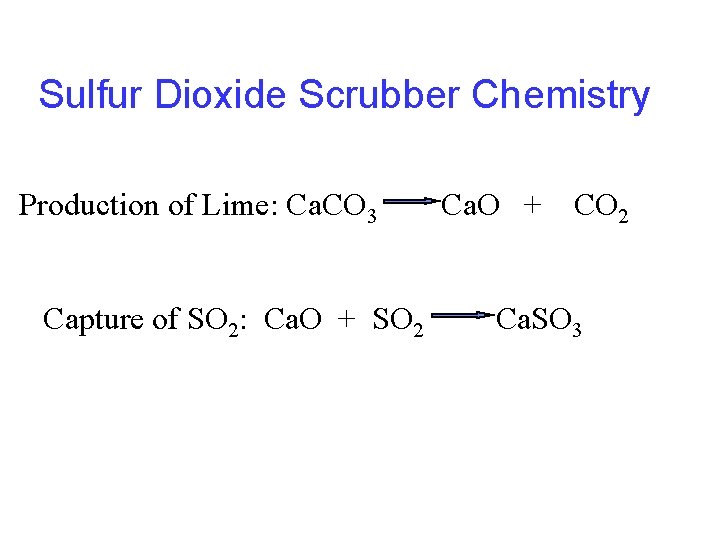
Sulfur Dioxide Scrubber Chemistry Production of Lime: Ca. CO 3 Capture of SO 2: Ca. O + SO 2 Ca. O + CO 2 Ca. SO 3

Sulfur Dioxide Scrubber

Answers to Problems: Lecture 16 1. Pideal = 57. 745 atm; Pvd. V = 45. 751 atm