Gases Laws Notes Pressure Pressure force per unit







- Slides: 26

Gases Laws Notes

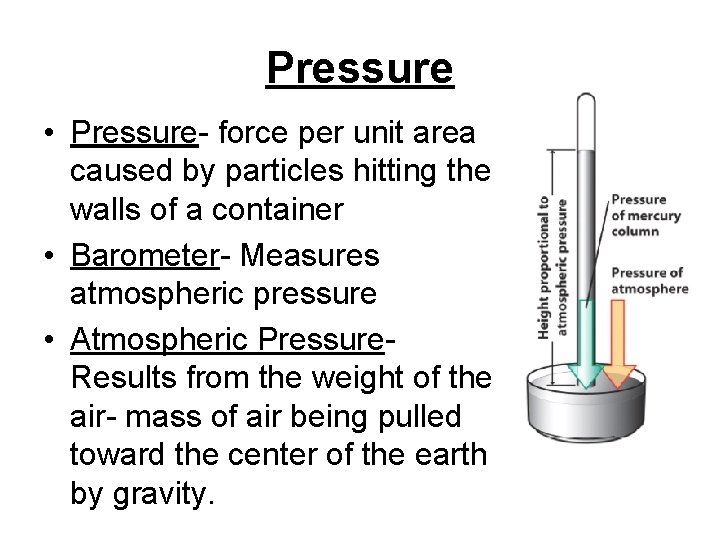
Pressure • Pressure- force per unit area caused by particles hitting the walls of a container • Barometer- Measures atmospheric pressure • Atmospheric Pressure. Results from the weight of the air- mass of air being pulled toward the center of the earth by gravity.

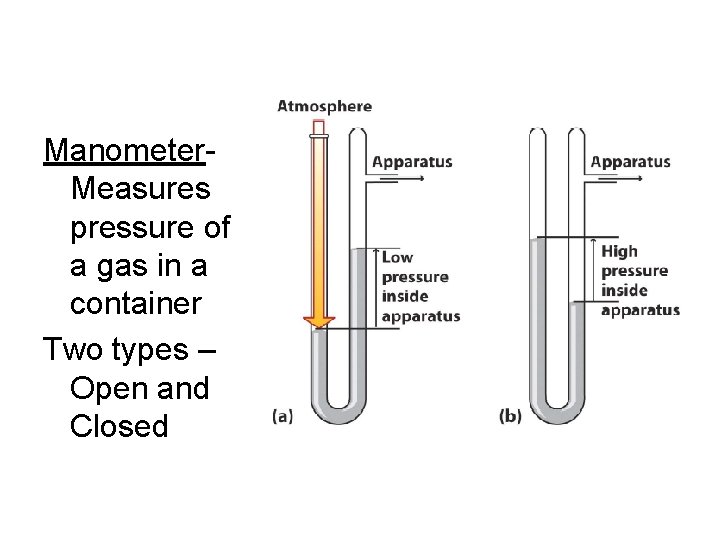
Manometer. Measures pressure of a gas in a container Two types – Open and Closed

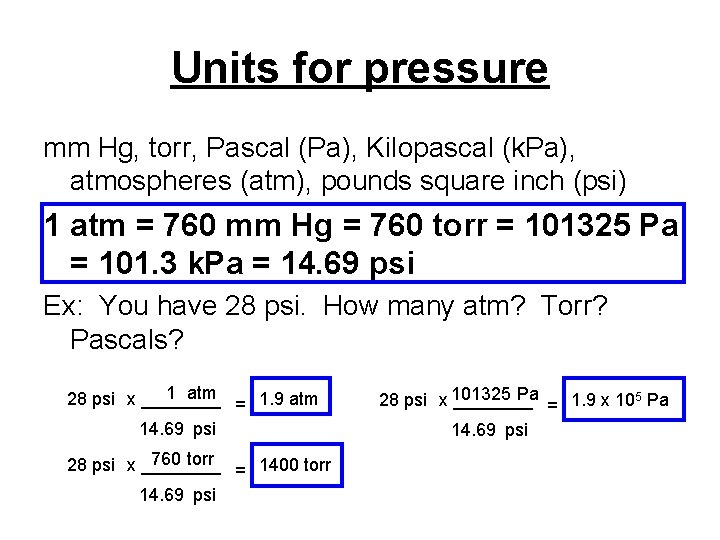
Units for pressure mm Hg, torr, Pascal (Pa), Kilopascal (k. Pa), atmospheres (atm), pounds square inch (psi) 1 atm = 760 mm Hg = 760 torr = 101325 Pa = 101. 3 k. Pa = 14. 69 psi Ex: You have 28 psi. How many atm? Torr? Pascals? 1 atm 28 psi x ____ = 1. 9 atm 14. 69 psi 760 torr 28 psi x ____ = 1400 torr 14. 69 psi Pa 5 28 psi x 101325 ____ = 1. 9 x 10 Pa 14. 69 psi

Learning Check • How many mm. Hg are 8. 92 atm?

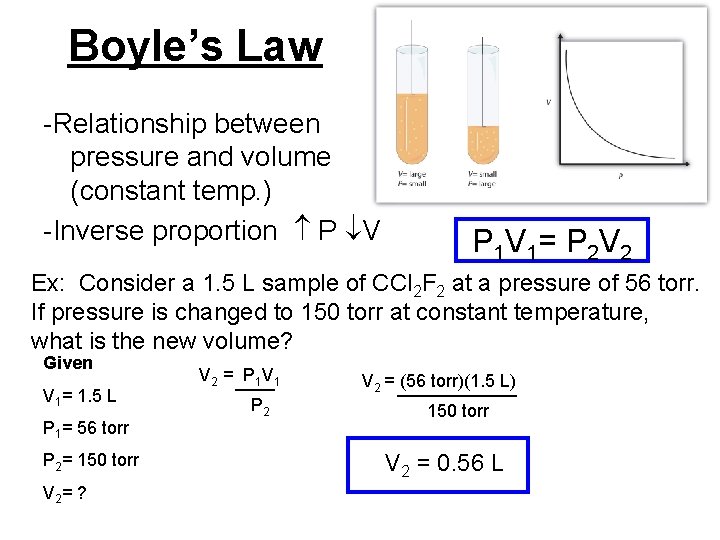
Boyle’s Law -Relationship between pressure and volume (constant temp. ) -Inverse proportion P V P 1 V 1= P 2 V 2 Ex: Consider a 1. 5 L sample of CCl 2 F 2 at a pressure of 56 torr. If pressure is changed to 150 torr at constant temperature, what is the new volume? Given V 1= 1. 5 L P 1= 56 torr P 2= 150 torr V 2= ? V 2 = ____ P 1 V 1 P 2 V 2 = ______ (56 torr)(1. 5 L) 150 torr V 2 = 0. 56 L

Learning Check • Boyle’s Law variables: • Is Boyle’s Law an inverse or direct relationship? • Boyle’s Law constants: • Boyle’s Law formula:

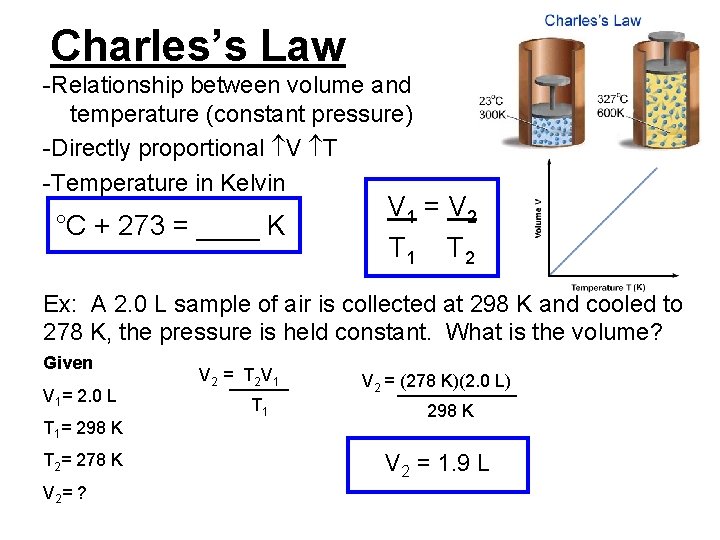
Charles’s Law -Relationship between volume and temperature (constant pressure) -Directly proportional V T -Temperature in Kelvin °C + 273 = ____ K V 1 = V 2 T 1 T 2 Ex: A 2. 0 L sample of air is collected at 298 K and cooled to 278 K, the pressure is held constant. What is the volume? Given V 1= 2. 0 L T 1= 298 K T 2= 278 K V 2= ? V 2 =______ T 2 V 1 T 1 V 2 = ______ (278 K)(2. 0 L) 298 K V 2 = 1. 9 L

Learning Check • Charles’ Law variables: • Is Charles’ Law an inverse or direct relationship? • Charles’ Law constants: • Charles’ Law formula:

Gay-Lussac’s Law -Relationship between pressure and temperature (constant volume) -Directly proportional P T -Temperature in Kelvin P 1 = P 2 T 1 °C + 273 = ____ K T 2 Ex: A mylar balloon is filled with helium gas to a pressure of 107 k. Pa when the temperature is 22 °C. If the temperature changes to is 45 °C, what will be the pressure of helium in the balloon? Given P 1= 107 k. Pa T 1= 22 °C + 273 = 295 K T 2= 45 °C + 273 = 318 K P 2= ? P 2 =______ T 2 P 1 T 1 P 2 = _______ (318 K)(107 k. Pa) 295 K P 2 = 115 k. Pa

Learning Check • Gay-Lussac’s Law variables: • Is Gay-Lussac’s Law an inverse or direct relationship? • Gay-Lussac’s Law constants: • Gay-Lussac’s Law formula:

Avogadro’s Law -Relationship between volume and moles (constant temperature and pressure) -Directly proportional V n V 1 = V 2 n 1 n 2 n = # of moles Ex: 12. 2 L sample contains 0. 50 moles O 2. If O 2 is converted to O 3, what will the volume be? 3 O 2 2 O 3 Given V 1= 12. 2 L n 1= 0. 50 mol V 2 =______ n 2 V 1 n 1 2 mol O 3 n 2= 0. 50 mol O 2 x ____ = 0. 33 mol O 2 V =? 2 V 2 = (0. 33 mol)(12. 2 L) _______ 0. 50 mol V 2 = 8. 1 L

Learning Check • Avogadro’s Law variables: • Is Avogadro’s Law an inverse or direct relationship? • Avogadro’s Law constants: • Avogadro’s Law formula:

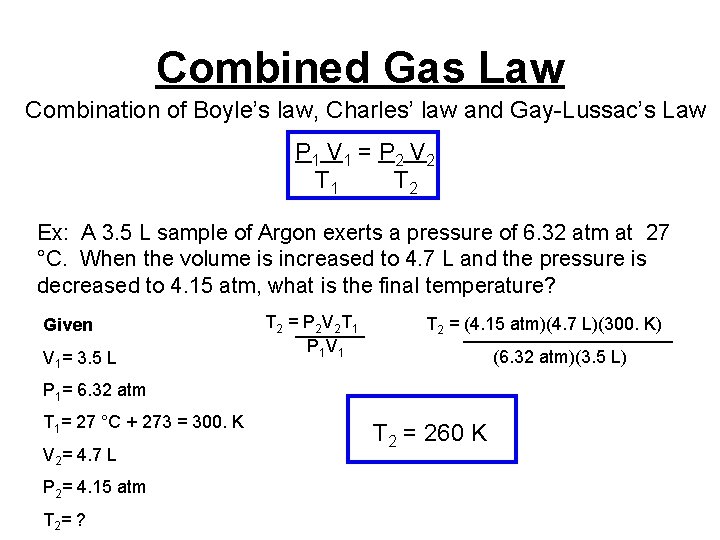
Combined Gas Law Combination of Boyle’s law, Charles’ law and Gay-Lussac’s Law P 1 V 1 = P 2 V 2 T 1 T 2 Ex: A 3. 5 L sample of Argon exerts a pressure of 6. 32 atm at 27 °C. When the volume is increased to 4. 7 L and the pressure is decreased to 4. 15 atm, what is the final temperature? Given V 1= 3. 5 L T 2 =_______ P 2 V 2 T 1 P 1 V 1 T 2 = (4. 15 atm)(4. 7 L)(300. K) ___________ (6. 32 atm)(3. 5 L) P 1= 6. 32 atm T 1= 27 °C + 273 = 300. K V 2= 4. 7 L P 2= 4. 15 atm T 2 = ? T 2 = 260 K

Learning Check • • Combined Gas Law variables: Combined Gas Law constants: Combined Gas Law formula: A gas is heated from 263. 0 K to 298. 0 K and the volume is increased from 24. 0 liters to 35. 0 liters by moving a large piston within a cylinder. If the original pressure was 1. 00 atm, what would the final pressure be?

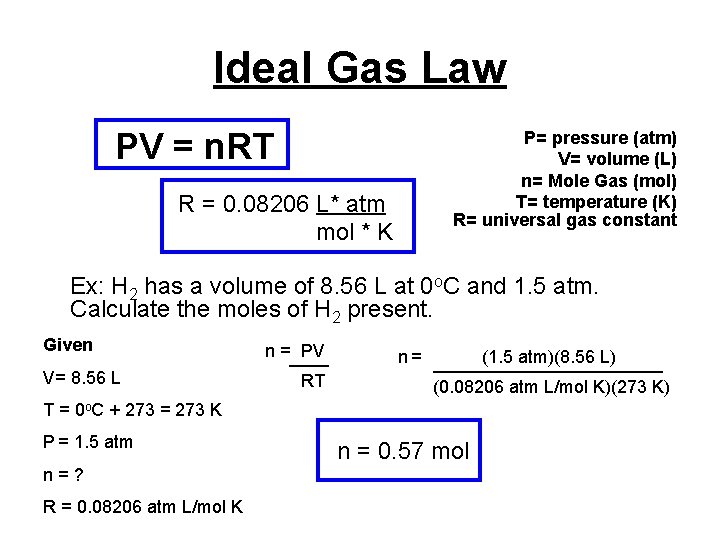
Ideal Gas Law PV = n. RT R = 0. 08206 L* atm mol * K P= pressure (atm) V= volume (L) n= Mole Gas (mol) T= temperature (K) R= universal gas constant Ex: H 2 has a volume of 8. 56 L at 0 o. C and 1. 5 atm. Calculate the moles of H 2 present. Given V= 8. 56 L n =____ PV RT n = ____________ (1. 5 atm)(8. 56 L) (0. 08206 atm L/mol K)(273 K) T = 0 o. C + 273 = 273 K P = 1. 5 atm n=? R = 0. 08206 atm L/mol K n = 0. 57 mol

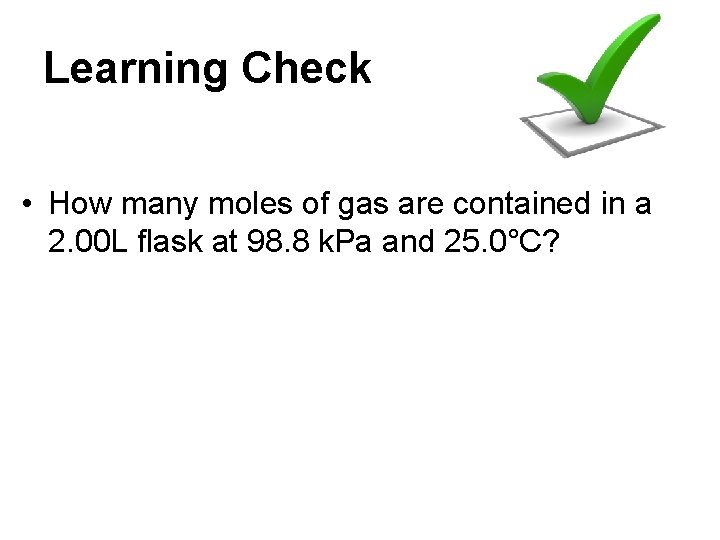
Learning Check • How many moles of gas are contained in a 2. 00 L flask at 98. 8 k. Pa and 25. 0°C?

Dalton’s Law of Partial Pressures Partial Pressure – pressure that a gas would exert if it alone in the container. Ptotal = P 1 + P 2 + P 3… Ex: A container holds three gases: oxygen, carbon dioxide, and helium. The partial pressures of the three gases are 2. 00 atm, 3. 00 atm, and 4. 00 atm, respectively. What is the total pressure inside the container? Given P 1= 2. 00 atm P 2= 3. 00 atm P 3= 4. 00 atm Ptotal= ? Ptotal = P 1 + P 2 + P 3 Ptotal = 2. 00 atm + 3. 00 atm + 4. 00 atm Ptotal = 9. 00 atm

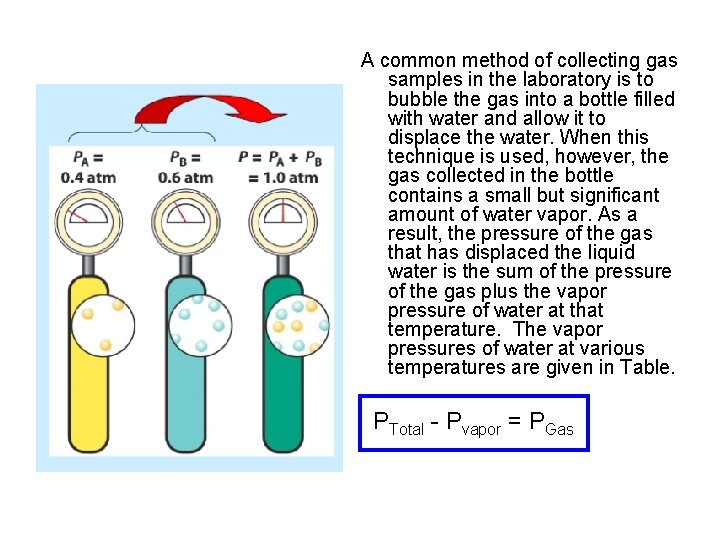
A common method of collecting gas samples in the laboratory is to bubble the gas into a bottle filled with water and allow it to displace the water. When this technique is used, however, the gas collected in the bottle contains a small but significant amount of water vapor. As a result, the pressure of the gas that has displaced the liquid water is the sum of the pressure of the gas plus the vapor pressure of water at that temperature. The vapor pressures of water at various temperatures are given in Table. PTotal - Pvapor = PGas

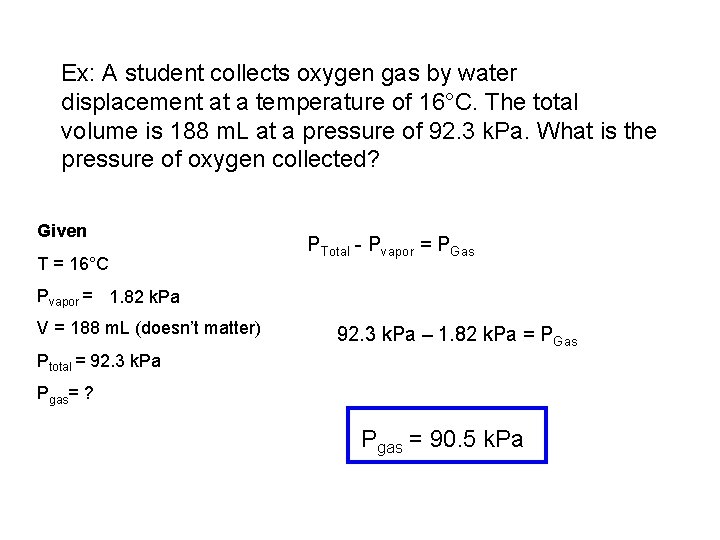
Ex: A student collects oxygen gas by water displacement at a temperature of 16°C. The total volume is 188 m. L at a pressure of 92. 3 k. Pa. What is the pressure of oxygen collected? Given T = 16°C PTotal - Pvapor = PGas Pvapor = 1. 82 k. Pa V = 188 m. L (doesn’t matter) 92. 3 k. Pa – 1. 82 k. Pa = PGas Ptotal = 92. 3 k. Pa Pgas= ? Pgas = 90. 5 k. Pa

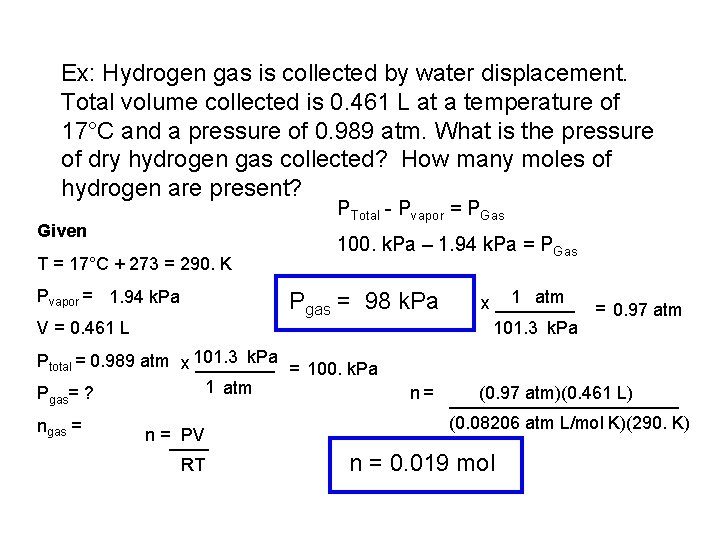
Ex: Hydrogen gas is collected by water displacement. Total volume collected is 0. 461 L at a temperature of 17°C and a pressure of 0. 989 atm. What is the pressure of dry hydrogen gas collected? How many moles of hydrogen are present? PTotal - Pvapor = PGas Given T = 17°C + 273 = 290. K Pvapor = 1. 94 k. Pa 100. k. Pa – 1. 94 k. Pa = PGas Pgas = 98 k. Pa V = 0. 461 L k. Pa Ptotal = 0. 989 atm x 101. 3 ____ = 100. k. Pa 1 atm P =? gas ngas = n =____ PV RT 1 atm x ____ = 0. 97 atm 101. 3 k. Pa n = ____________ (0. 97 atm)(0. 461 L) (0. 08206 atm L/mol K)(290. K) n = 0. 019 mol

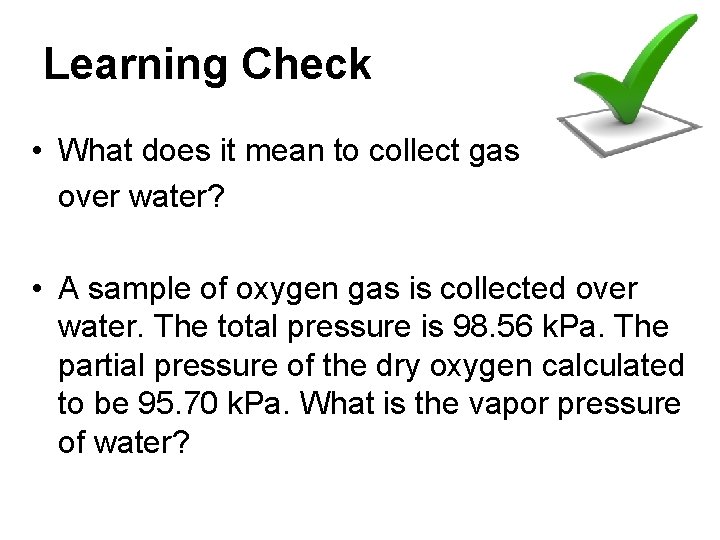
Learning Check • What does it mean to collect gas over water? • A sample of oxygen gas is collected over water. The total pressure is 98. 56 k. Pa. The partial pressure of the dry oxygen calculated to be 95. 70 k. Pa. What is the vapor pressure of water?

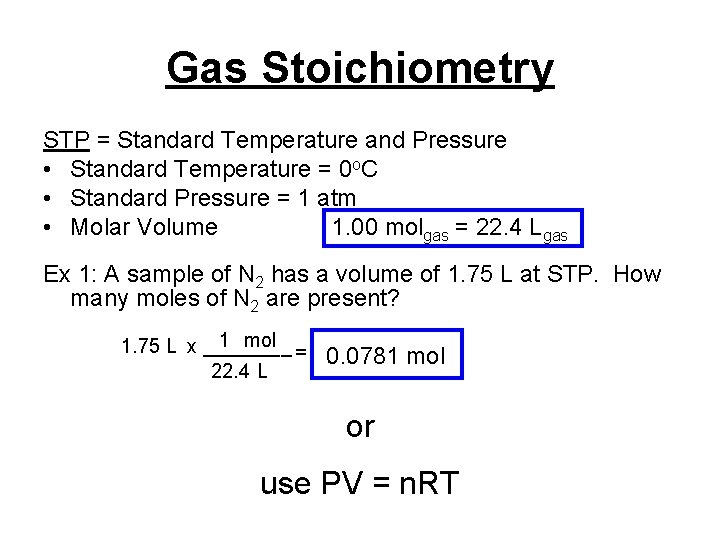
Gas Stoichiometry STP = Standard Temperature and Pressure • Standard Temperature = 0 o. C • Standard Pressure = 1 atm • Molar Volume 1. 00 molgas = 22. 4 Lgas Ex 1: A sample of N 2 has a volume of 1. 75 L at STP. How many moles of N 2 are present? 1 mol 1. 75 L x ____ = 22. 4 L 0. 0781 mol or use PV = n. RT

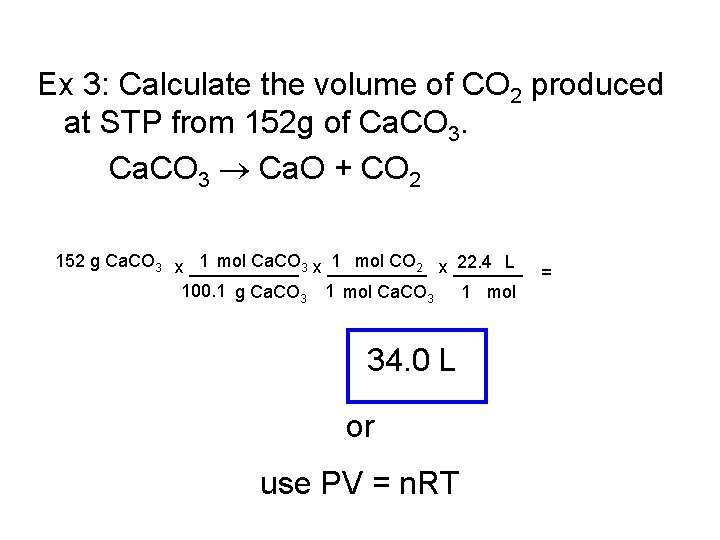
Ex 3: Calculate the volume of CO 2 produced at STP from 152 g of Ca. CO 3 Ca. O + CO 2 152 g Ca. CO 3 x ______ 1 mol Ca. CO 3 x _____ 1 mol CO 2 x _______ 22. 4 L = 100. 1 g Ca. CO 3 1 mol 34. 0 L or use PV = n. RT

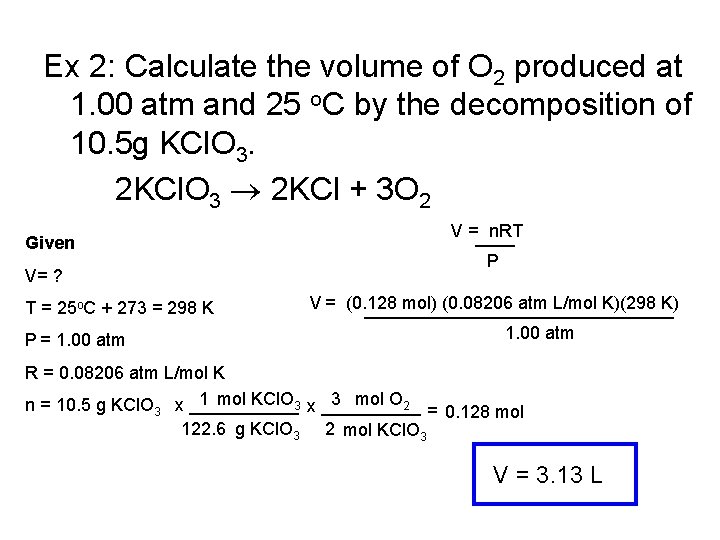
Ex 2: Calculate the volume of O 2 produced at 1. 00 atm and 25 o. C by the decomposition of 10. 5 g KCl. O 3. 2 KCl. O 3 2 KCl + 3 O 2 Given V= ? T = 25 o. C + 273 = 298 K P = 1. 00 atm V =____ n. RT P V = (0. 128 mol) (0. 08206 atm L/mol K)(298 K) ________________ 1. 00 atm R = 0. 08206 atm L/mol K 1 mol KCl. O 3 x _____ 3 mol O 2 n = 10. 5 g KCl. O 3 x ______ = 0. 128 mol 122. 6 g KCl. O 3 2 mol KCl. O 3 V = 3. 13 L

CHALLENGE! • What would the value of the Gas Constant, R, be with the following units? 1) k. Pa*L mol*K 2) atm*L kmol*K