UNIT 8 Gas Laws KINETIC MOLECULAR THEORY Particles


























- Slides: 66

UNIT 8 Gas Laws!

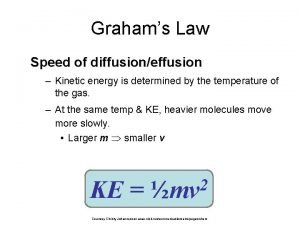
KINETIC MOLECULAR THEORY Particles in an ideal gas… have no volume. have elastic collisions. are in constant, random, straight-line motion. don’t attract or repel each other. have an avg. KE directly related to Kelvin temperature.

REAL GASES Particles in a REAL gas… have their own volume attract each other Gas behavior is most ideal… at low pressures at high temperatures in nonpolar atoms/molecules

THE NATURE OF GASES Gases expand to fill their containers Gases are fluids – they flow Gases have low density 1/1000 the density of the equivalent liquid or solid Gases are compressible Gases effuse and diffuse

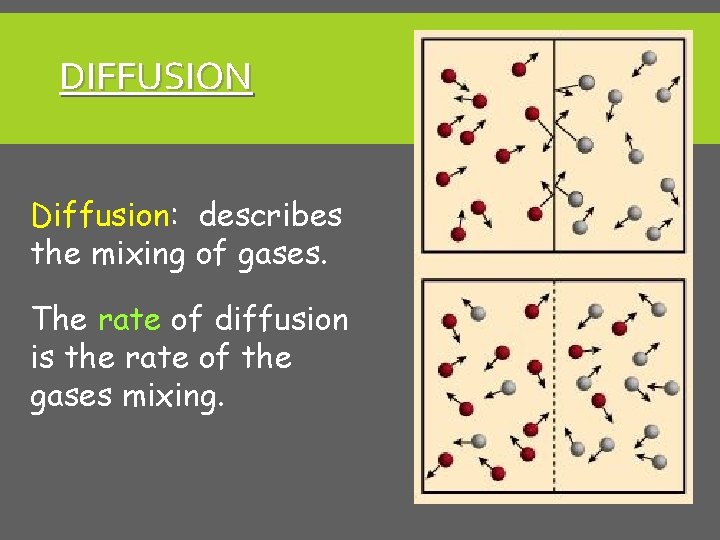
DIFFUSION Diffusion: describes the mixing of gases. The rate of diffusion is the rate of the gases mixing.

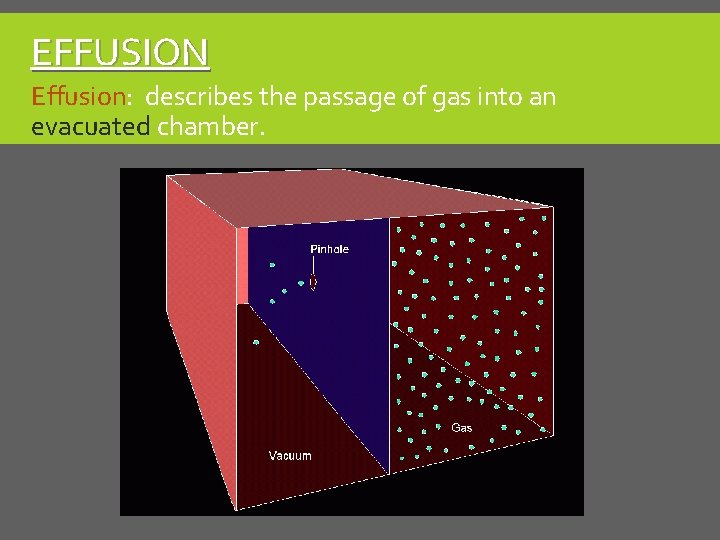
EFFUSION Effusion: describes the passage of gas into an evacuated chamber.

KINETIC MOLECULAR THEORY -MODEL FOR IDEAL GASES ARE IMAGINARY GASES THAT PERFECTLY FIT ALL OF THE ASSUMPTIONS OF THE KINETIC MOLECULAR THEORY. Gases consist of tiny particles that are far apart relative to their size. Collisions between gas particles, between other particles, and the walls of the container are elastic collisions

KINETIC MOLECULAR THEORY (CONT. ) -MODEL FOR IDEAL GASES ARE IMAGINARY GASES THAT PERFECTLY FIT ALL OF THE ASSUMPTIONS OF THE KINETIC MOLECULAR THEORY. No kinetic energy is lost in elastic collisions There are no forces of attraction between gas particles The average kinetic energy of gas particles depends on temperature, not on the identity of the particle.

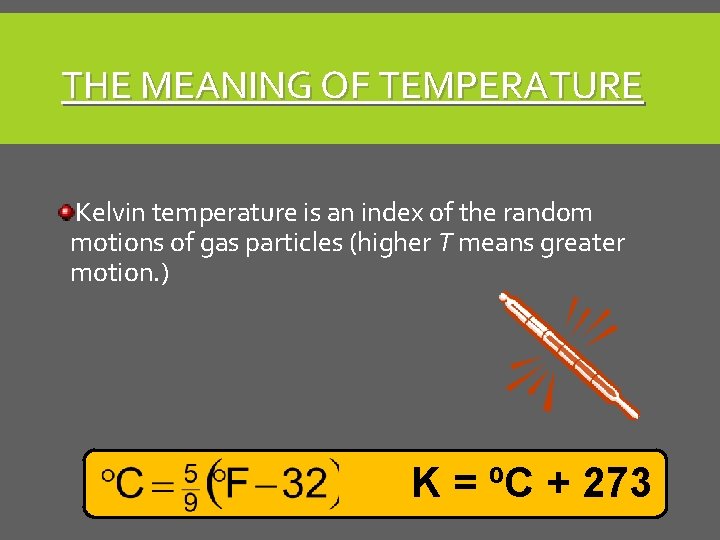
THE MEANING OF TEMPERATURE Kelvin temperature is an index of the random motions of gas particles (higher T means greater motion. ) K = ºC + 273

PRESSURE Is caused by the collisions of molecules with the walls of a container is equal to force/unit area SI units = Newton/meter 2 = 1 Pascal (Pa) 1 standard atmosphere = 101, 325 Pa 1 standard atmosphere = 1 atm = 760 mm Hg = 760 torr

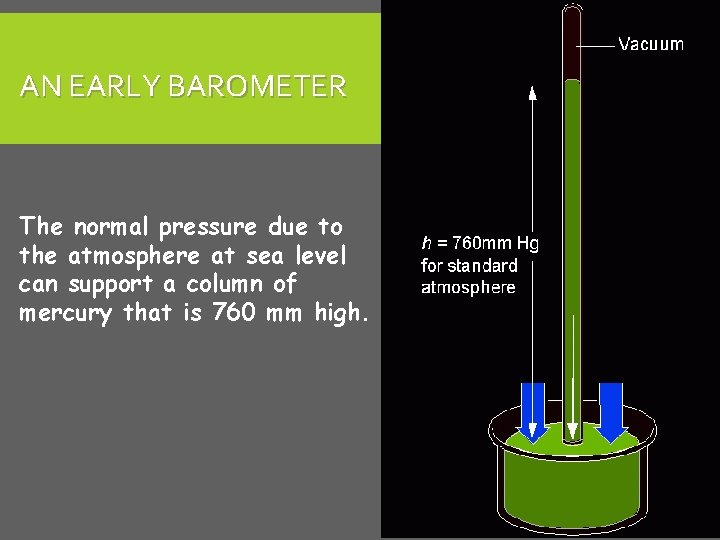
MEASURING PRESSURE The first device for measuring atmospheric pressure was developed by Evangelista Torricelli during the 17 th century. The device was called a “barometer” Baro = weight Meter = measure

AN EARLY BAROMETER The normal pressure due to the atmosphere at sea level can support a column of mercury that is 760 mm high.

Practice Problem The average atmospheric pressure in Denver, Colorado is 0. 830 atm. Express this pressure in (a) millimeters of Hg and (b) k. Pa.

Practice Problem The average atmospheric pressure in Denver, Colorado is 0. 830 atm. Express this pressure in (a) millimeters of Hg and (b) k. Pa. 760 mm Hg = 1 atm 101. 325 k. Pa = 1 atm 760 torr = 1 am a. 631 mm Hg b. 84. 1 k. Pa

More Practice Problem A) 0. 985 atm to mm Hg B) 642 Torr to k. Pa C) 849 Torr to atm D) 4. 00 atm to Torr

More Practice Problem A) 0. 985 atm to mm Hg 749 mm Hg B) 642 Torr to k. Pa 85. 6 k. Pa C) 849 Torr to atm 1. 12 atm D) 4. 00 atm to Torr 3040 Torr

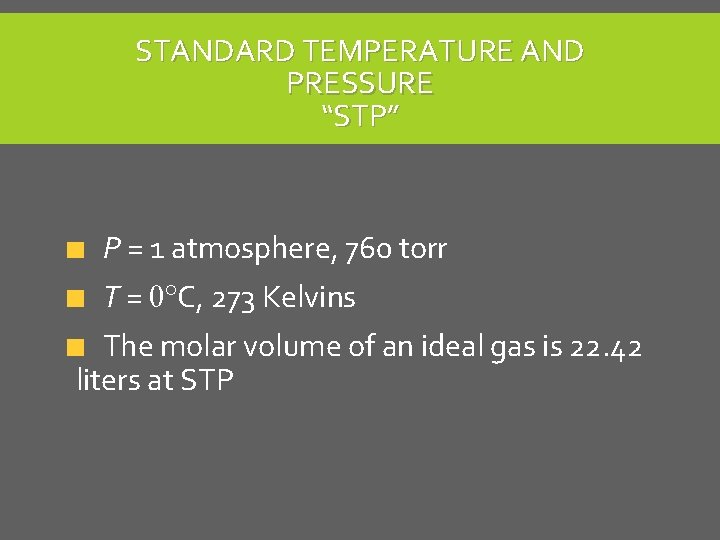
STANDARD TEMPERATURE AND PRESSURE “STP” P = 1 atmosphere, 760 torr T = 0 C, 273 Kelvins The molar volume of an ideal gas is 22. 42 liters at STP

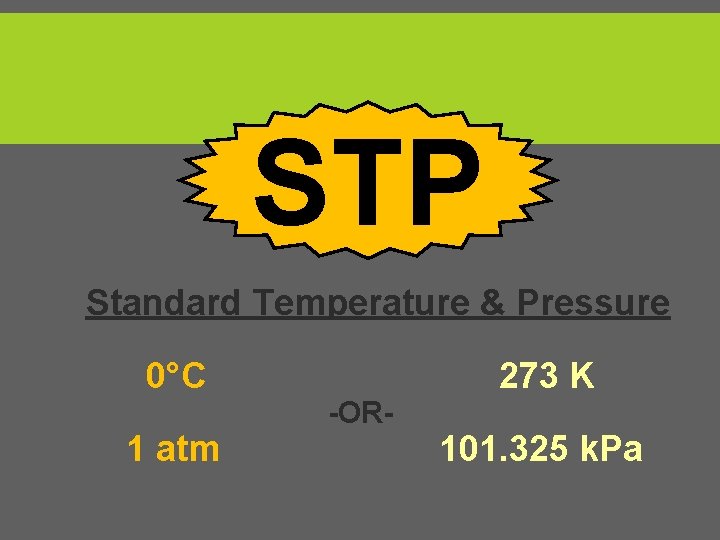
STP Standard Temperature & Pressure 0°C 1 atm 273 K -OR- 101. 325 k. Pa

CONVERTING CELSIUS TO KELVIN Gas law problems involving temperature require that the temperature be in KELVINS! Kelvins = C + 273 °C = Kelvins - 273 Absolute zero-the temperature where all molecular motion ceases – O K

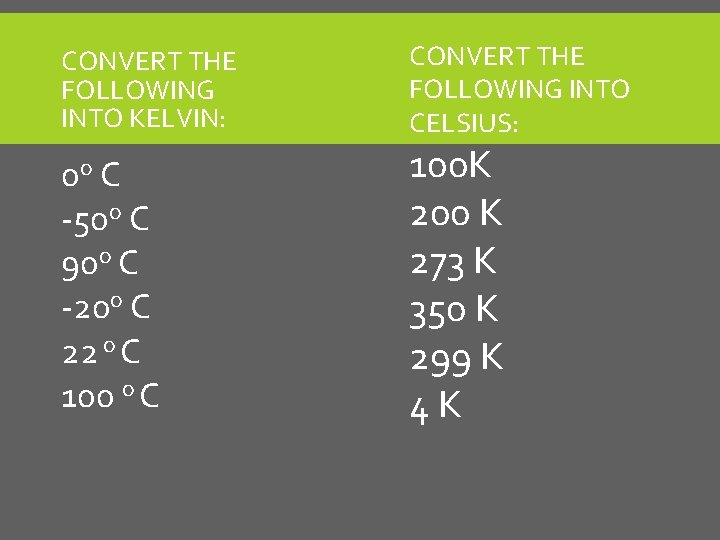
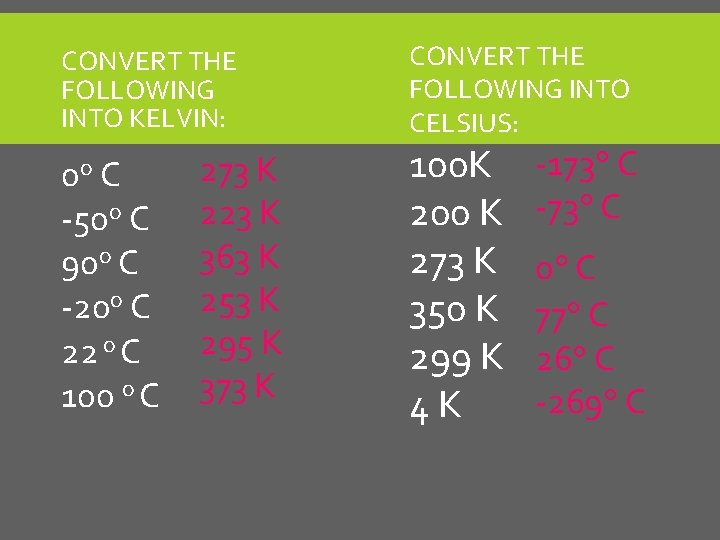
CONVERT THE FOLLOWING INTO KELVIN: 0 o C -50 o C 90 o C -20 o C 22 o C 100 o C CONVERT THE FOLLOWING INTO CELSIUS: 100 K 200 K 273 K 350 K 299 K 4 K

CONVERT THE FOLLOWING INTO KELVIN: 0 o C -50 o C 90 o C -20 o C 22 o C 100 o C 273 K 223 K 363 K 253 K 295 K 373 K CONVERT THE FOLLOWING INTO CELSIUS: 100 K 200 K 273 K 350 K 299 K 4 K -173 C -73 C 0 C 77 C 26 C -269 C

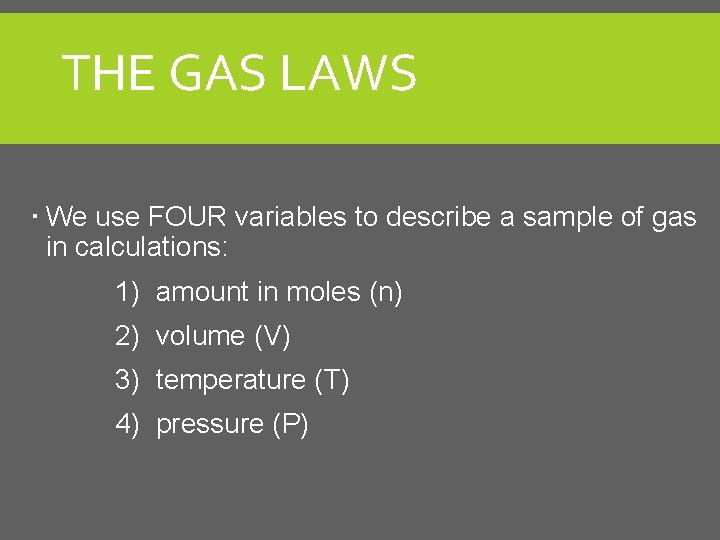
THE GAS LAWS We use FOUR variables to describe a sample of gas in calculations: 1) amount in moles (n) 2) volume (V) 3) temperature (T) 4) pressure (P)

THE GAS LAWS These four variables (moles, V, T, and P) are related through some simple gas laws that show one of the variables changes as a second variables changes, and the other two remain constant

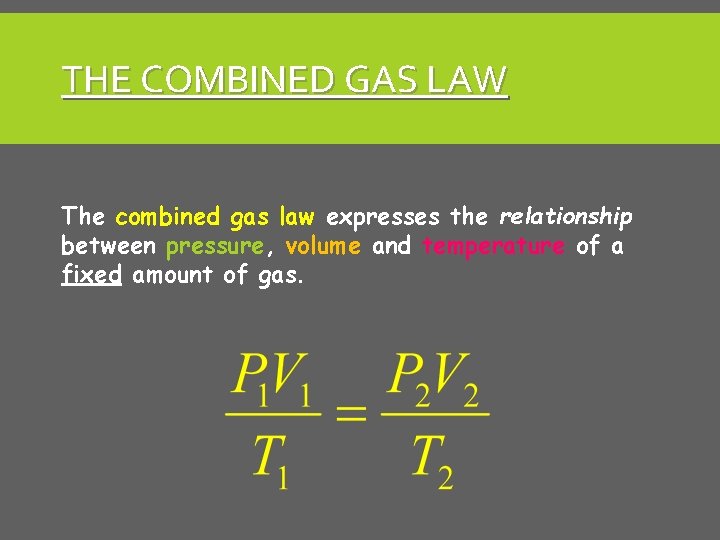
THE COMBINED GAS LAW The combined gas law expresses the relationship between pressure, volume and temperature of a fixed amount of gas.

Practice Problem A helium-filled balloon has a volume of 50. 0 L at 25 C and 1. 08 atm. What volume will it have a 0. 855 atm and 10. 0 C? (remember T must be in Kelvin)

Practice Problem A helium-filled balloon has a volume of 50. 0 L at 25 C and 1. 08 atm. What volume will it have a 0. 855 atm and 10. 0 C? P 1 V 1/T 1 = P 2 V 2/T 2 P 1 V 1 T 2/P 2 T 1 = V 2 = 60. 0 L He

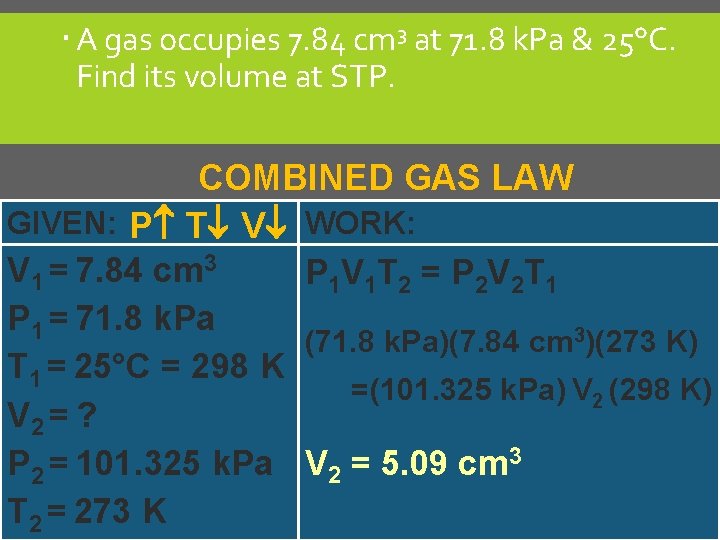
A gas occupies 7. 84 cm 3 at 71. 8 k. Pa & 25°C. Find its volume at STP. COMBINED GAS LAW GIVEN: P T V WORK: V 1 = 7. 84 cm 3 P 1 V 1 T 2 = P 2 V 2 T 1 P 1 = 71. 8 k. Pa (71. 8 k. Pa)(7. 84 cm 3)(273 K) T 1 = 25°C = 298 K =(101. 325 k. Pa) V 2 (298 K) V 2 = ? P 2 = 101. 325 k. Pa V 2 = 5. 09 cm 3 T 2 = 273 K C. Johannesson

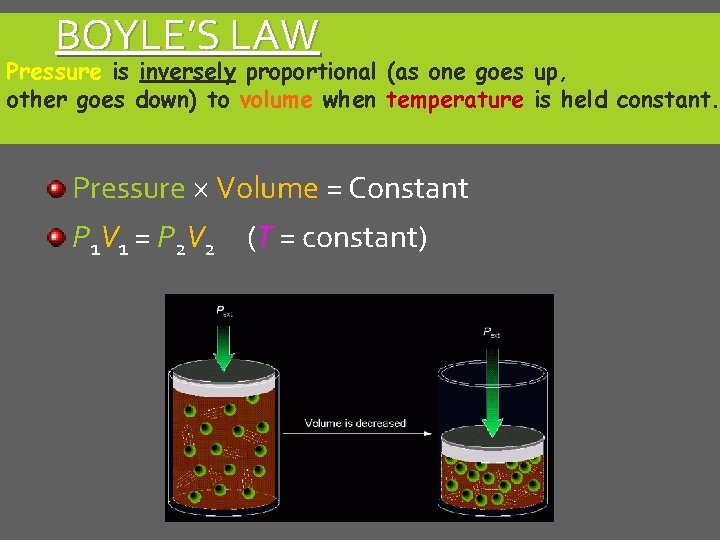
BOYLE’S LAW Pressure is inversely proportional (as one goes up, other goes down) to volume when temperature is held constant. Pressure ´ Volume = Constant P 1 V 1 = P 2 V 2 (T = constant)

BOYLE’S LAW P 1 V 1 = P 2 V 2 Inverse Relationship T is constant If one goes up, the other goes down If one goes down, the other goes up

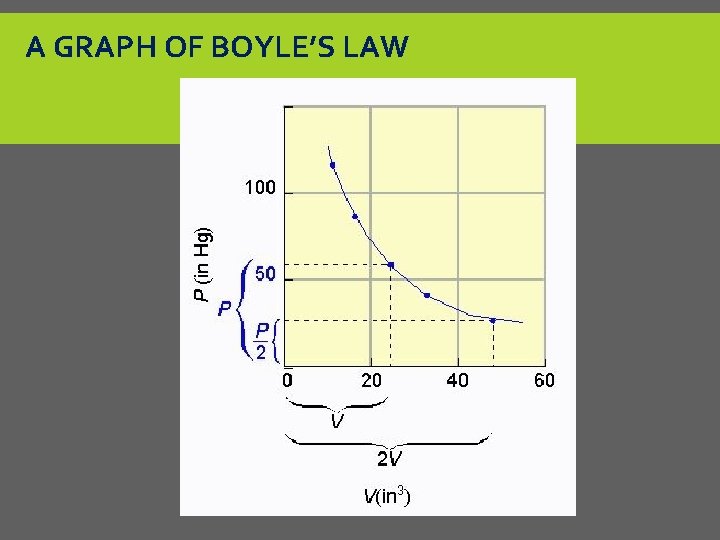
A GRAPH OF BOYLE’S LAW

Practice Problem A sample of oxygen gas has a volume of 150. 0 m. L when its pressure is 0. 947 atm. What will the volume of the gas be at a pressure of 0. 987 atm if the temperature remains constant? Pressure ´ Volume = Constant P 1 V 1 = P 2 V 2 (T = constant) (P 1 V 1)/P 2 = V 2 = 144 m. L O 2

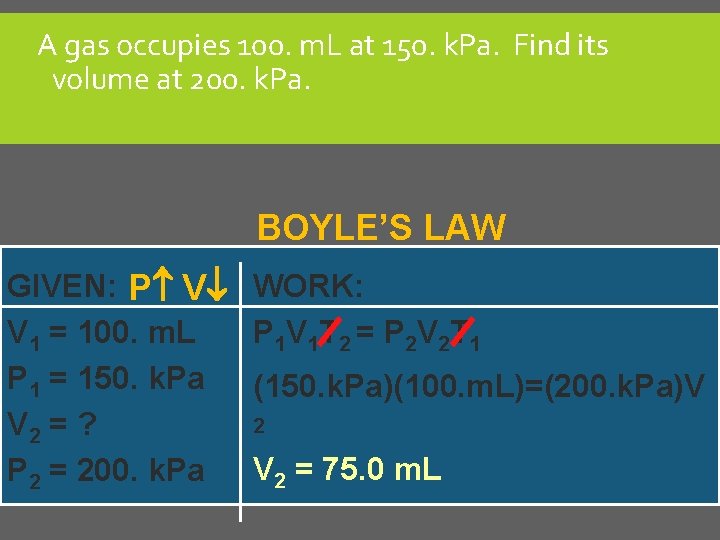
A gas occupies 100. m. L at 150. k. Pa. Find its volume at 200. k. Pa. BOYLE’S LAW GIVEN: P V V 1 = 100. m. L P 1 = 150. k. Pa V 2 = ? P 2 = 200. k. Pa WORK: P 1 V 1 T 2 = P 2 V 2 T 1 (150. k. Pa)(100. m. L)=(200. k. Pa)V 2 = 75. 0 m. L

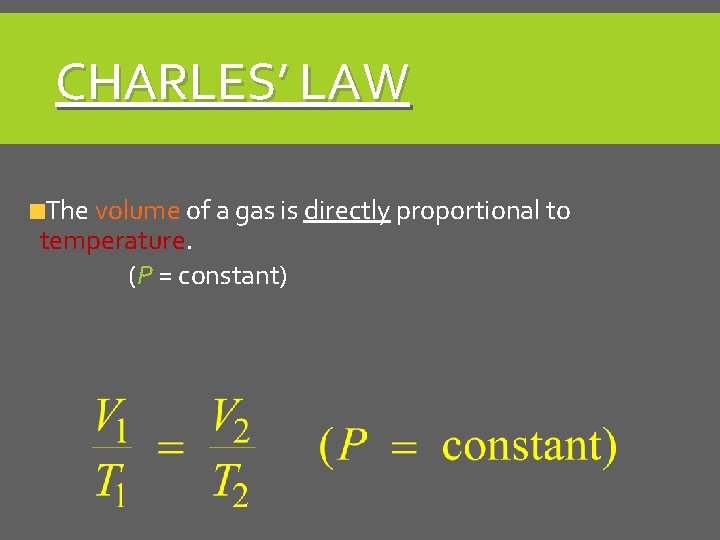
CHARLES’ LAW The volume of a gas is directly proportional to temperature. (P = constant)

CHARLES’ LAW V 1/T 1 = V 2/T 2 Direct Relationship P is constant Temp in Kelvin!!! (°C + 273) As one goes up, so does the other As one goes down, so does the other higher temp = more kinetic energy, more collisions, so volume increases

Practice Problem A sample of neon gas occupies a volume of 752 m. L at 25 C. What volume will the gas occupy at 50 C if the pressure remains constant? V 1 T 2/T 1 = V 2 = 815 m. L Ne

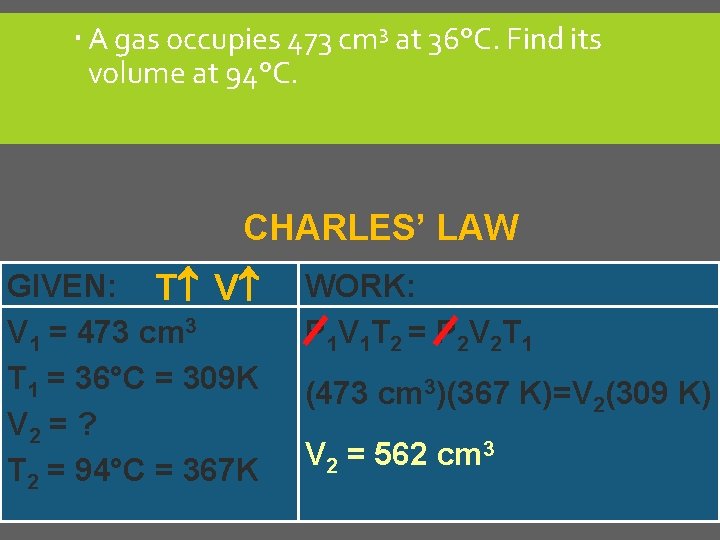
A gas occupies 473 cm 3 at 36°C. Find its volume at 94°C. CHARLES’ LAW GIVEN: T V V 1 = 473 cm 3 T 1 = 36°C = 309 K V 2 = ? T 2 = 94°C = 367 K WORK: P 1 V 1 T 2 = P 2 V 2 T 1 (473 cm 3)(367 K)=V 2(309 K) V 2 = 562 cm 3 C. Johannesson

SOME VIDS: https: //www. youtube. com/watch? feature=endscreen&v=Pg. Xc. S wb. URBw&NR=1 https: //www. youtube. com/watch? v=tp. Zm-Kv 1 m 4 w Explain on your own sheet of paper, using your newly gained knowledge of pressure, volume, and temperature what is occurring in these videos.

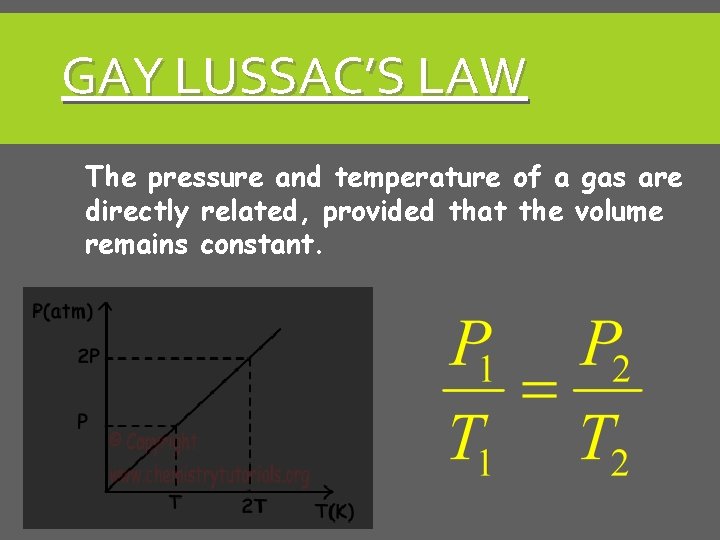
GAY LUSSAC’S LAW The pressure and temperature of a gas are directly related, provided that the volume remains constant.

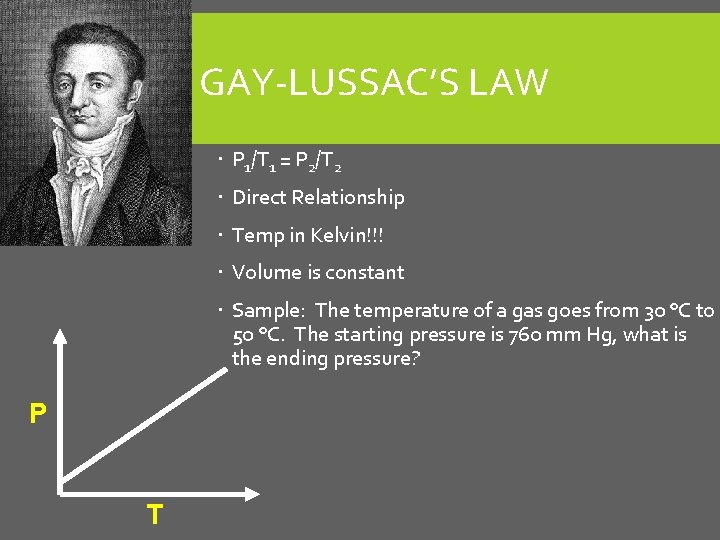
GAY-LUSSAC’S LAW P 1/T 1 = P 2/T 2 Direct Relationship Temp in Kelvin!!! Volume is constant Sample: The temperature of a gas goes from 30 °C to 50 °C. The starting pressure is 760 mm Hg, what is the ending pressure? P T

Practice Problem The gas in a container is at a pressure of 3. 00 atm at 25 C. Directions on the container warn the user not to keep it in a place where the temperature exceeds 52 C. What would the gas pressure in the container be at 52 C?

Practice Problem The gas in a container is at a pressure of 3. 00 atm at 25 C. Directions on the container warn the user not to keep it in a place where the temperature exceeds 52 C. What would the gas pressure in the container be at 52 C? P 1/T 1 = P 2/T 2 P 1 T 2/T 1 = P 2 =3. 27 atm

A gas’ pressure is 765 torr at 23°C. At what temperature will the pressure be 560. torr? GAY-LUSSAC’S LAW GIVEN: P T WORK: P 1 = 765 torr T 1 = 23°C = 296 K P 2 = 560. torr T 2 = ? P 1 V 1 T 2 = P 2 V 2 T 1 (765 torr)T 2 = (560. torr)(309 K) T 2 = 226 K = -47°C C. Johannesson

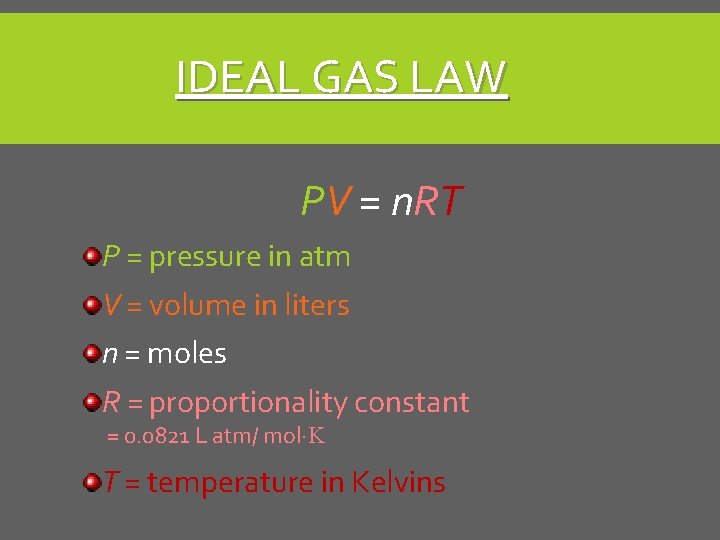
IDEAL GAS LAW PV = n. RT P = pressure in atm V = volume in liters n = moles R = proportionality constant = 0. 0821 L atm/ mol·K T = temperature in Kelvins

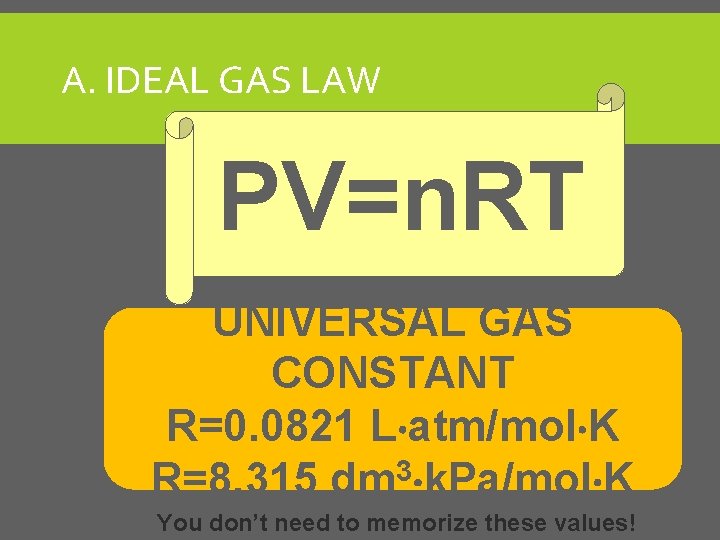
A. IDEAL GAS LAW PV=n. RT UNIVERSAL GAS CONSTANT R=0. 0821 L atm/mol K R=8. 315 dm 3 k. Pa/mol K You don’t need to memorize these values!

SAMPLE PROBLEMS… 1. You have 4 moles of helium gas at 1. 5 atm and 25 L, what is the temperature? 2. How many moles of argon gas are present in 500 m. L at 3 atm and 30 ºC?

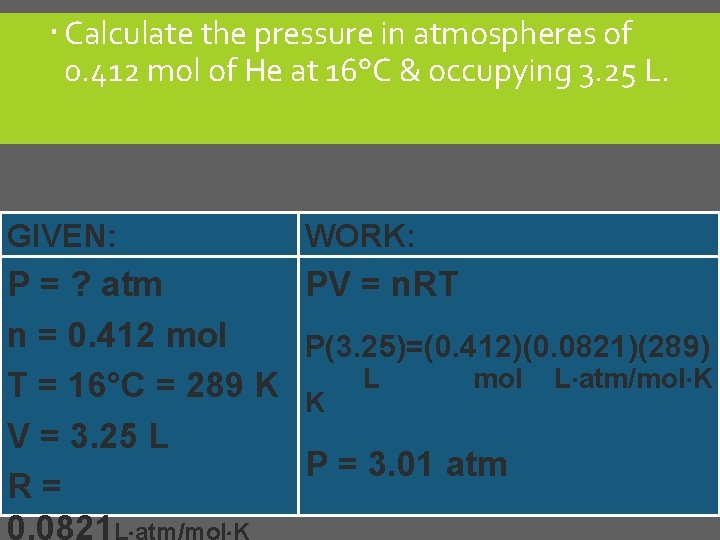
Calculate the pressure in atmospheres of 0. 412 mol of He at 16°C & occupying 3. 25 L. GIVEN: WORK: P = ? atm PV = n. RT n = 0. 412 mol P(3. 25)=(0. 412)(0. 0821)(289) mol L atm/mol K T = 16°C = 289 K K L V = 3. 25 L P = 3. 01 atm R= 0. 0821 L atm/mol K

Find the volume of 85 g of O 2 at 25°C and 104. 5 k. Pa. GIVEN: WORK: V=? 85 g 1 mol = 2. 7 mol n = 85 g = 2. 7 mol 32. 00 g T = 25°C = 298 K PV = n. RT P = 104. 5 k. Pa (104. 5)V=(2. 7) (8. 315) (298) k. Pa mol dm 3 k. Pa/mol K R = 8. 315 V = 64 dm 3 k. Pa/mol K C. Johannesson

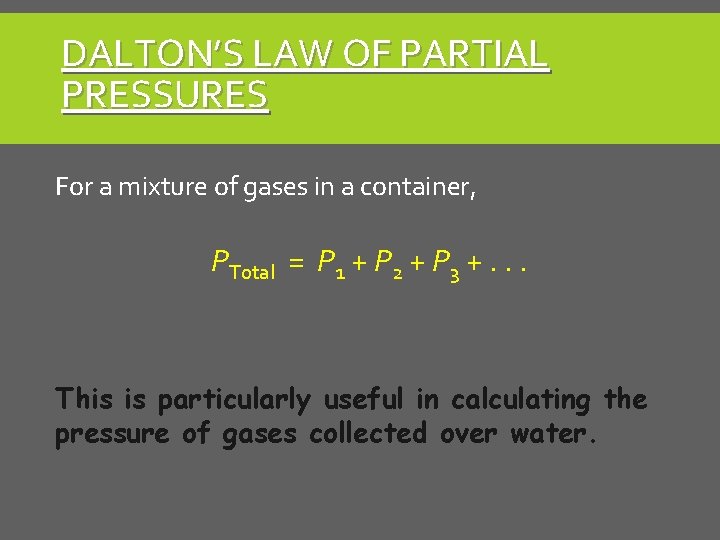
DALTON’S LAW OF PARTIAL PRESSURES For a mixture of gases in a container, PTotal = P 1 + P 2 + P 3 +. . . This is particularly useful in calculating the pressure of gases collected over water.

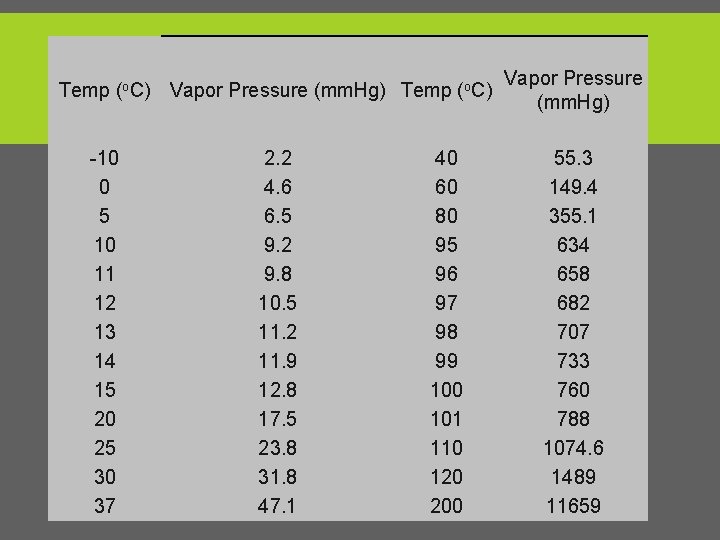
Temp (o. C) Vapor Pressure (mm. Hg) Temp (o. C) -10 0 5 10 11 12 13 14 15 20 25 30 37 2. 2 4. 6 6. 5 9. 2 9. 8 10. 5 11. 2 11. 9 12. 8 17. 5 23. 8 31. 8 47. 1 40 60 80 95 96 97 98 99 100 101 110 120 200 Vapor Pressure (mm. Hg) 55. 3 149. 4 355. 1 634 658 682 707 733 760 788 1074. 6 1489 11659

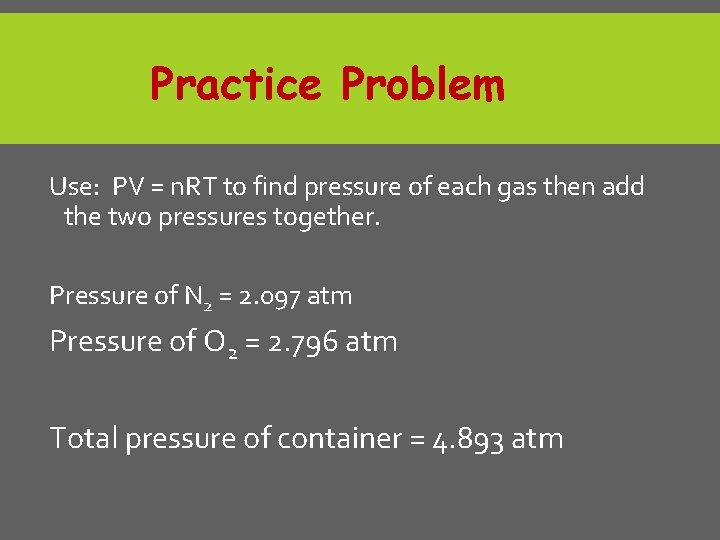
Practice Problem If I place 3 moles of N 2 and 4 moles of O 2 in a 35 m. L container at a temperature of 25°C, what will the pressure of the resulting mixture of gases be?

Practice Problem Use: PV = n. RT to find pressure of each gas then add the two pressures together. Pressure of N 2 = 2. 097 atm Pressure of O 2 = 2. 796 atm Total pressure of container = 4. 893 atm

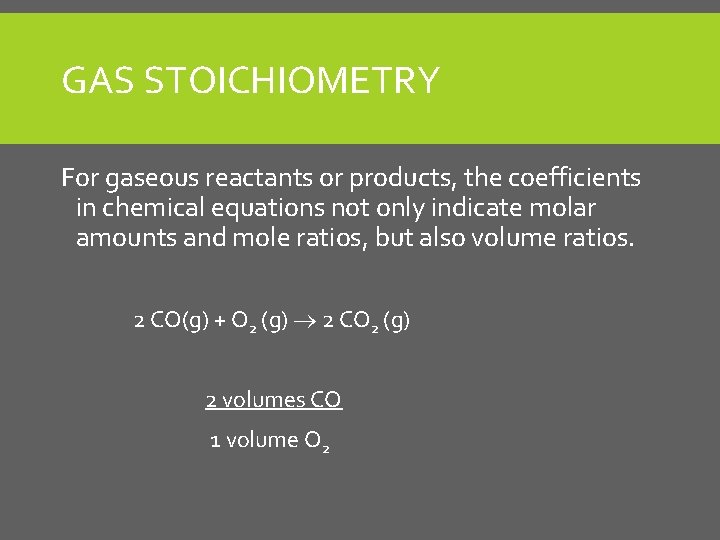
GAS STOICHIOMETRY For gaseous reactants or products, the coefficients in chemical equations not only indicate molar amounts and mole ratios, but also volume ratios. 2 CO(g) + O 2 (g) 2 CO 2 (g) 2 volumes CO 1 volume O 2

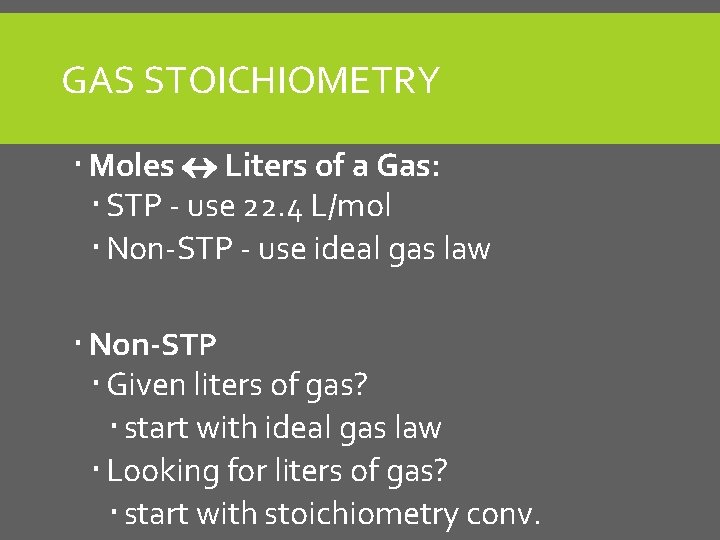
GAS STOICHIOMETRY Moles Liters of a Gas: STP - use 22. 4 L/mol Non-STP - use ideal gas law Non-STP Given liters of gas? start with ideal gas law Looking for liters of gas? start with stoichiometry conv.

Practice Problem Propane, C 3 H 8 is a gas that is sometimes used as a fuel for cooking and heating. C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O(g) a. What will be the volume, in L, of oxygen required for the complete combustion of 0. 350 L of propane? b. What will be the volume of CO 2 produced in the reaction? Assume all volume measurements are made at the same temperature and pressure.

Practice Problem Propane, C 3 H 8 is a gas that is sometimes used as a fuel for cooking and heating. C 2 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O(g) a. What will be the volume, in L, of oxygen required for the complete combustion of 0. 350 L of propane? 1. 75 L O 2 b. What will be the volume of CO 2 produced in the reaction? Assume all volume measurements are made at the same temperature and pressure. 1. 05 L CO 2

Practice Problem 2 H 2(G) + O 2(G) 2 H 2 O(L) How many liters of water can be made from 55 grams of oxygen gas and an excess of hydrogen at STP?

Practice Problem 2 H 2(G) + O 2(G) 2 H 2 O(L) How many liters of water can be made from 55 grams of oxygen gas and an excess of hydrogen at STP? = 77 L H 2 O

STANDARD MOLAR VOLUME Equal volumes of all gases at the same temperature and pressure contain the same number of molecules. - Amedeo Avogadro

STANDARD MOLAR VOLUME

GAS STOICHIOMETRY 1 mole = 22. 4 liters At STP

Practice Problem 1. What volume does 0. 0685 mol of gas occupy at STP? 2. What quantity of gas, in moles, is contained in 2. 21 L at STP?

Practice Problem 1. What volume does 0. 0685 mol of gas occupy at STP? 1. 53 L 2. What quantity of gas, in moles, is contained in 2. 21 L at STP? 0. 0987 mol

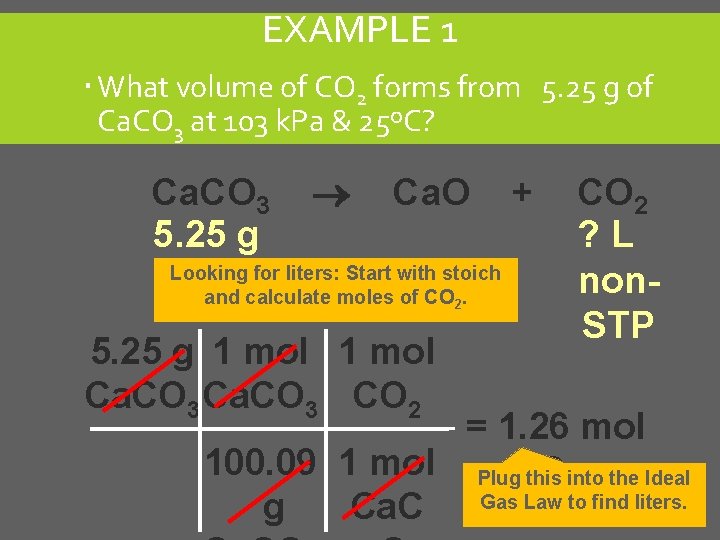
EXAMPLE 1 What volume of CO 2 forms from 5. 25 g of Ca. CO 3 at 103 k. Pa & 25ºC? Ca. CO 3 5. 25 g Ca. O Looking for liters: Start with stoich and calculate moles of CO 2. 5. 25 g 1 mol Ca. CO 3 CO 2 + CO 2 ? L non. STP = 1. 26 mol 100. 09 1 mol Plug. CO this 2 into the Ideal Gas Law to find liters. g Ca. C

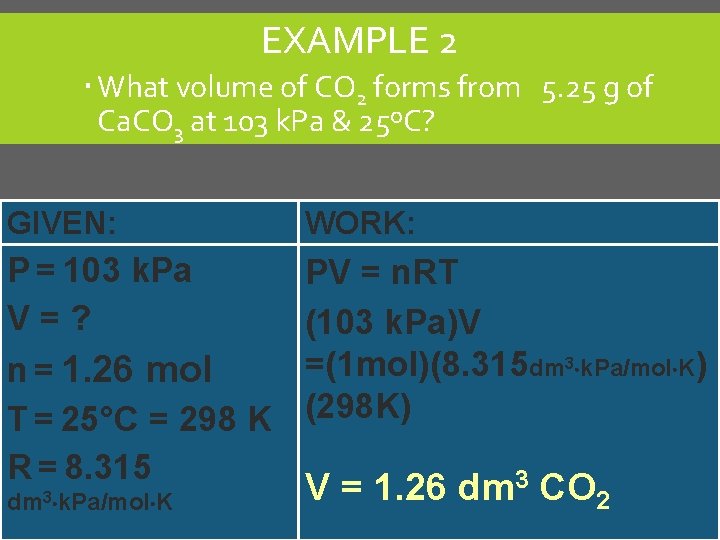
EXAMPLE 2 What volume of CO 2 forms from 5. 25 g of Ca. CO 3 at 103 k. Pa & 25ºC? GIVEN: WORK: P = 103 k. Pa V=? n = 1. 26 mol T = 25°C = 298 K R = 8. 315 PV = n. RT (103 k. Pa)V =(1 mol)(8. 315 dm 3 k. Pa/mol K) (298 K) dm 3 k. Pa/mol K V = 1. 26 dm 3 CO 2 C. Johannesson

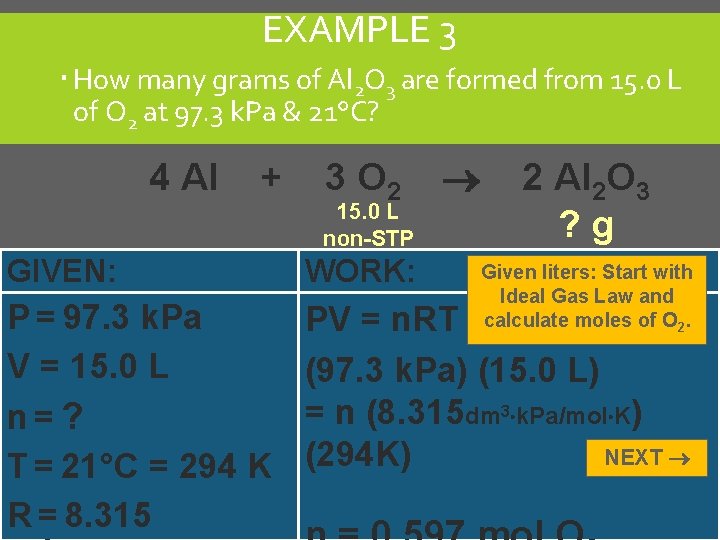
EXAMPLE 3 How many grams of Al 2 O 3 are formed from 15. 0 L of O 2 at 97. 3 k. Pa & 21°C? 4 Al + 3 O 2 15. 0 L non-STP 2 Al 2 O 3 ? g GIVEN: WORK: P = 97. 3 k. Pa V = 15. 0 L n=? T = 21°C = 294 K R = 8. 315 PV = n. RT (97. 3 k. Pa) (15. 0 L) = n (8. 315 dm 3 k. Pa/mol K) NEXT (294 K) Given liters: Start with Ideal Gas Law and calculate moles of O 2. C. Johannesson

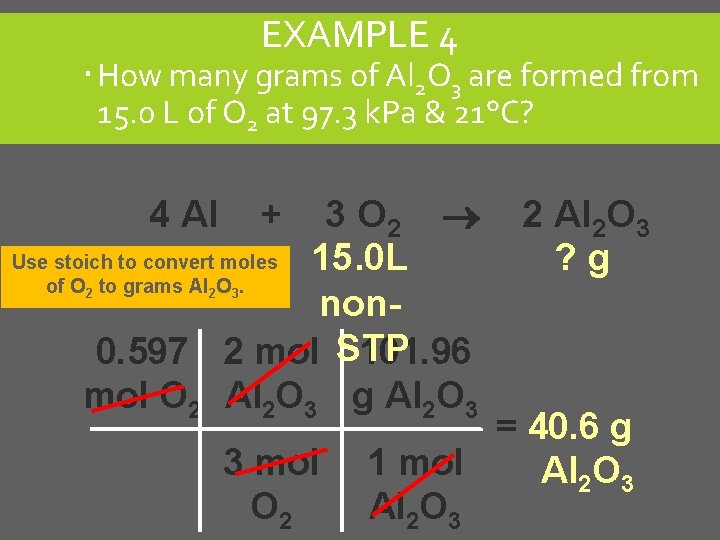
EXAMPLE 4 How many grams of Al 2 O 3 are formed from 15. 0 L of O 2 at 97. 3 k. Pa & 21°C? 3 O 2 Use stoich to convert moles 15. 0 L of O to grams Al O. non 0. 597 2 mol STP 101. 96 mol O 2 Al 2 O 3 g Al 2 O 3 4 Al 2 2 + 2 Al 2 O 3 ? g 3 3 mol O 2 = 40. 6 g 1 mol Al 2 O 3
 Buoyancyability
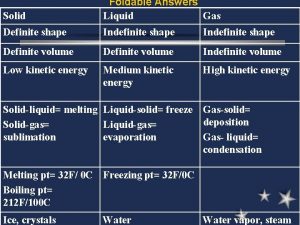
Buoyancyability Chapter 14 solids liquids and gases
Chapter 14 solids liquids and gases Kinetic molecular theory
Kinetic molecular theory Kinetic molecular theory volume
Kinetic molecular theory volume Adhesive force
Adhesive force Kinetic molecular theory of liquids
Kinetic molecular theory of liquids Postulates of kinetic molecular theory
Postulates of kinetic molecular theory Kinetic theory def
Kinetic theory def Theory vs hypothesis
Theory vs hypothesis Charles law in terms of kinetic molecular theory
Charles law in terms of kinetic molecular theory Kinetic theory of gases postulates
Kinetic theory of gases postulates Avogadro's law
Avogadro's law Postulates of kinetic theory of gases
Postulates of kinetic theory of gases Equation of state of real gas
Equation of state of real gas Kinetic molecular theory
Kinetic molecular theory Tenets of kinetic molecular theory
Tenets of kinetic molecular theory Properties of solid
Properties of solid Solid
Solid Postulate 1
Postulate 1 Solid liquid gas particles
Solid liquid gas particles Gas particles are separated by relatively large distances
Gas particles are separated by relatively large distances Chapter 21: temperature, heat, and expansion answer key
Chapter 21: temperature, heat, and expansion answer key Gas like mixture of charged particles
Gas like mixture of charged particles Difference between mot and vbt
Difference between mot and vbt Vbt theory
Vbt theory Valence bond theory and molecular orbital theory
Valence bond theory and molecular orbital theory Valence bond theory and molecular orbital theory
Valence bond theory and molecular orbital theory Valence bond theory
Valence bond theory What is a covalent bond simple definition
What is a covalent bond simple definition Ionic covalent metallic
Ionic covalent metallic Giant molecular structure vs simple molecular structure
Giant molecular structure vs simple molecular structure Useless laws weaken the necessary laws
Useless laws weaken the necessary laws Relation between pressure and kinetic energy of gas
Relation between pressure and kinetic energy of gas Kinetic energy of gas molecules
Kinetic energy of gas molecules Kinetic gas equation
Kinetic gas equation Gas laws crash course
Gas laws crash course Boyle's law indirect or direct
Boyle's law indirect or direct What are the empirical gas laws
What are the empirical gas laws What is the combined gas law
What is the combined gas law Bourdon gauge gas law
Bourdon gauge gas law Different gas laws
Different gas laws How hot air balloons operate gas laws
How hot air balloons operate gas laws Conceptual gas law questions
Conceptual gas law questions Ideal gas examples
Ideal gas examples Chapter 13 gas laws worksheet answer key
Chapter 13 gas laws worksheet answer key Boyle's gas law formula
Boyle's gas law formula Different gas laws
Different gas laws Combined gas laws
Combined gas laws Which gas law relates pressure and temperature
Which gas law relates pressure and temperature Which gas laws are inversely proportional
Which gas laws are inversely proportional Charles law formula
Charles law formula Ap chemistry gas laws
Ap chemistry gas laws Gas law graphic organizer
Gas law graphic organizer Gas laws hot air balloon
Gas laws hot air balloon Gay lussac's law in real life
Gay lussac's law in real life Kmt gas laws
Kmt gas laws Gas laws formula
Gas laws formula Empirical gas laws
Empirical gas laws Charles law
Charles law Ideal gas laws
Ideal gas laws Solid liquid gas foldable
Solid liquid gas foldable Unit 10, unit 10 review tests, unit 10 general test
Unit 10, unit 10 review tests, unit 10 general test Si unit for kinetic energy
Si unit for kinetic energy Si unit for kinetic energy
Si unit for kinetic energy The kinetic theory of matter states that
The kinetic theory of matter states that Kinetic theory of matter definition
Kinetic theory of matter definition Define kinetic theory of matter
Define kinetic theory of matter