GAS LAWS KINETIC MOLECULAR THEORY Particles in an
















- Slides: 26

GAS LAWS

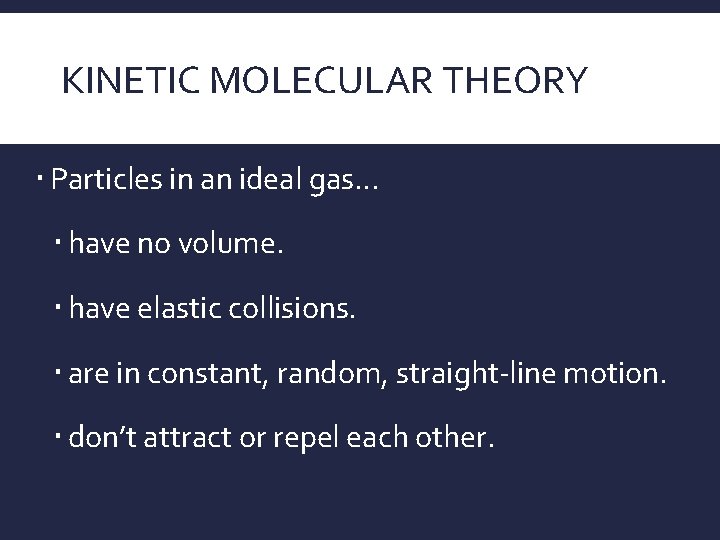
KINETIC MOLECULAR THEORY Particles in an ideal gas… have no volume. have elastic collisions. are in constant, random, straight-line motion. don’t attract or repel each other.

REAL GASES Particles in a REAL gas… have their own volume attract each other Gas behavior is most ideal… at low pressures at high temperatures in nonpolar atoms/molecules

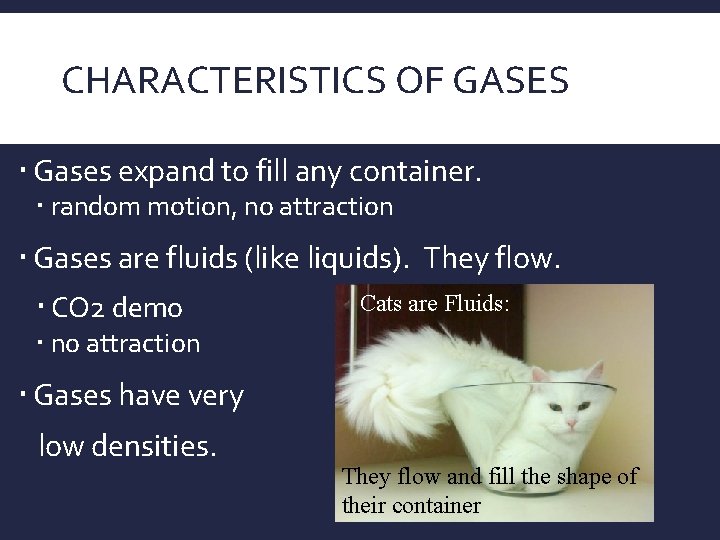
CHARACTERISTICS OF GASES Gases expand to fill any container. random motion, no attraction Gases are fluids (like liquids). They flow. CO 2 demo Cats are Fluids: no attraction Gases have very low densities. They flow and fill the shape of their container

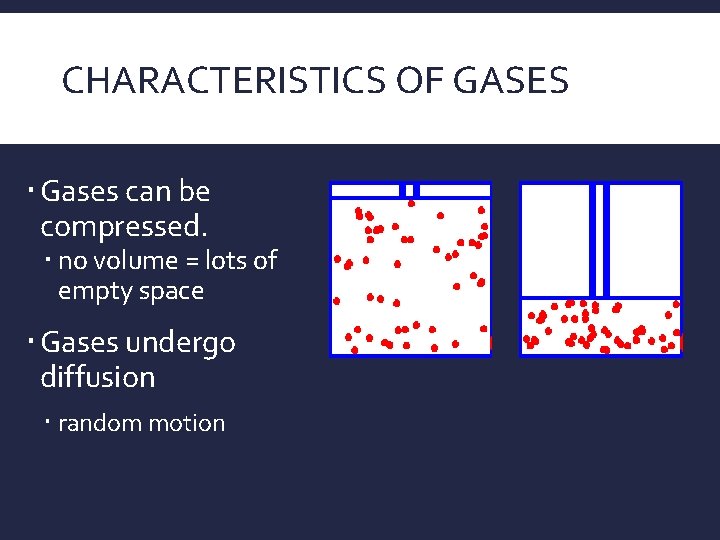
CHARACTERISTICS OF GASES Gases can be compressed. no volume = lots of empty space Gases undergo diffusion random motion

CHARACTERISTICS OF GASES

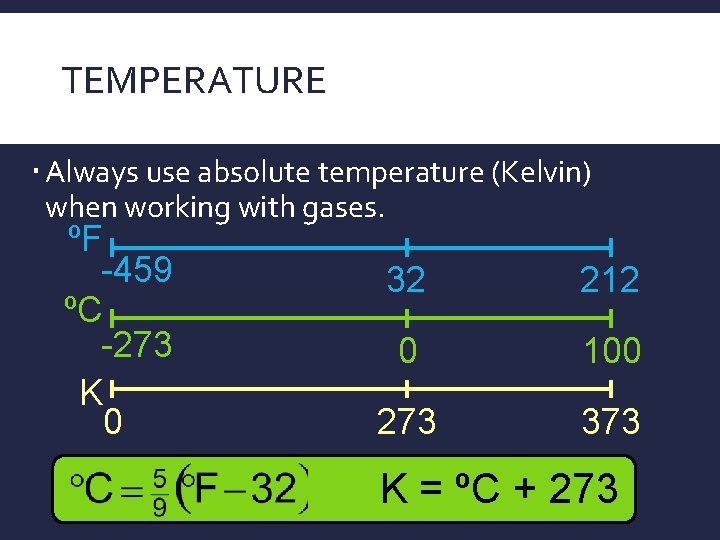
TEMPERATURE Always use absolute temperature (Kelvin) when working with gases. ºF -459 ºC -273 K 0 32 212 0 100 273 373 K = ºC + 273

PRESSURE Which shoes create the most pressure?

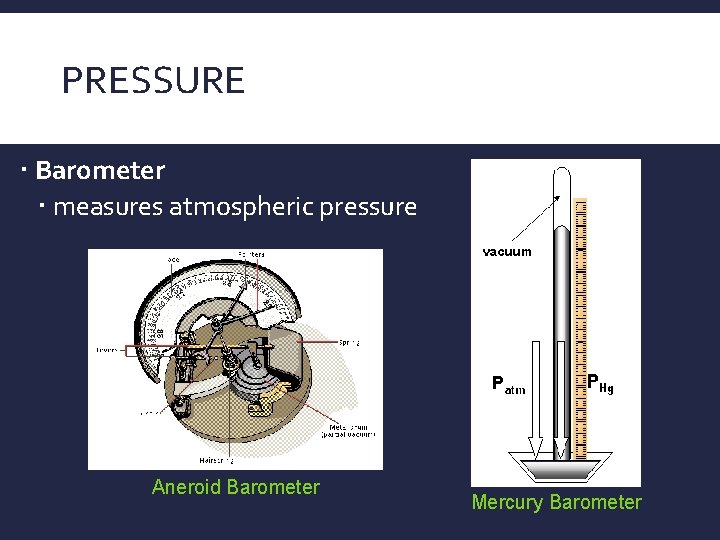
PRESSURE Barometer measures atmospheric pressure Aneroid Barometer Mercury Barometer

PRESSURE KEY UNITS AT SEA LEVEL 101. 325 k. Pa (kilopascal) 1 atm 760 mm Hg 760 torr 14. 7 psi

STP Standard Temperature & Pressure 1 atm 273 K

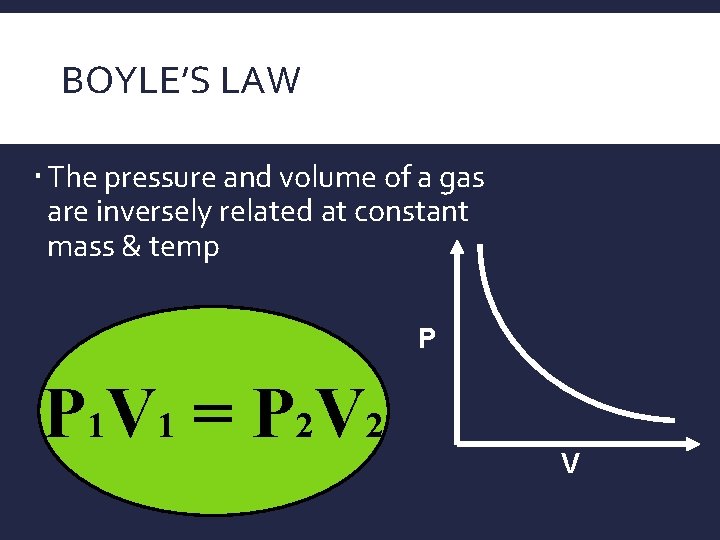
BOYLE’S LAW

BOYLE’S LAW The pressure and volume of a gas are inversely related at constant mass & temp P P 1 V 1 = P 2 V 2 V

PRACTICE QUESTION If a Helium tank that has an initial volume of 100 m. L and a pressure of 5 atm loses 50 m. L of Helium what is its new pressure? (5)(100) = P 2 (50) 500/50 = 10 P 2 = 10 atm

CHARLES’ LAW

CHARLES’ LAW The volume and absolute temperature (K) of a gas are directly related at constant mass & pressure V 1 = V 2 T 1 T 2 V T

PRACTICE QUESTION If a Helium balloon that has an intial volume of 100 m. L and a temperature of 240 K is placed in the sun where it heats to 300 K what is its new volume? (100) = V 2 (240) (300) 0. 42 = V 2 (300) V 2 = 125 m. L

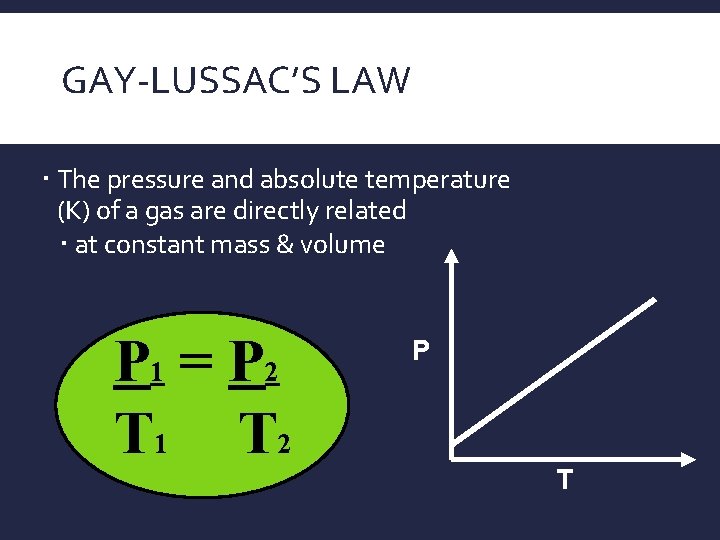
GAY-LUSSAC’S LAW

GAY-LUSSAC’S LAW The pressure and absolute temperature (K) of a gas are directly related at constant mass & volume P 1 = P 2 T 1 T 2 P T

PRACTICE QUESTION If a Helium tank that has an initial pressure of 5 atm and a temperature of 240 K is placed in the sun where it heats to 300 K what is the new pressure? (5) = P 2 (240) (300) 0. 021 = P 2 (300) P 2 = 6. 25 atm

DALTON’S LAW The total pressure of a mixture of gases equals the sum of the partial pressures of the individual gases. Ptotal = P 1 + P 2 +. . .

PRACTICE QUESTION A scuba tank is filled with a mixture of atmospheric gases. Nitrogen exerts a pressure of 70 atm. Oxygen gas exerts a pressure of 22 atm. Argon exerts a pressure of 4 atm. A fourth gas exerts an unknown amount of pressure. We know the total pressure of the tank is 98 atm. What is the partial pressure of the fourth gas? Ptotal = P 1 + P 2 + P 3 + P 4 98 = 70 +22 + 4 + p 4 98 = 96 + P 4 98 -96 = P 4 2 = P 4

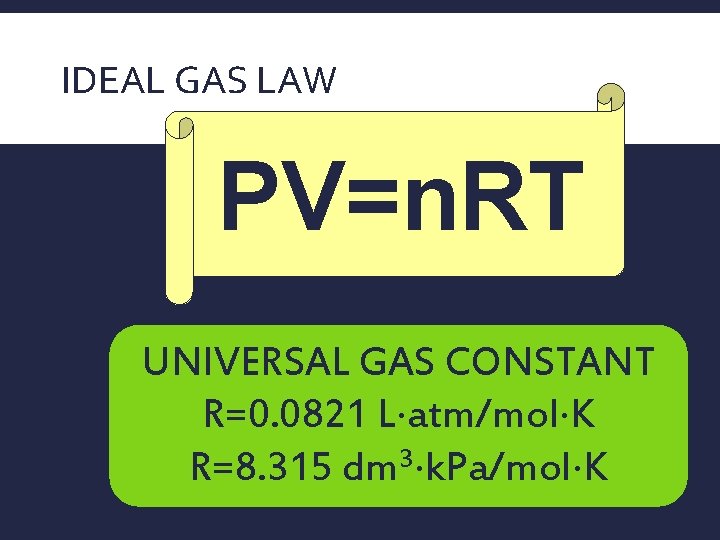
IDEAL GAS LAW PV=n. RT UNIVERSAL GAS CONSTANT R=0. 0821 L atm/mol K 3 R=8. 315 dm k. Pa/mol K

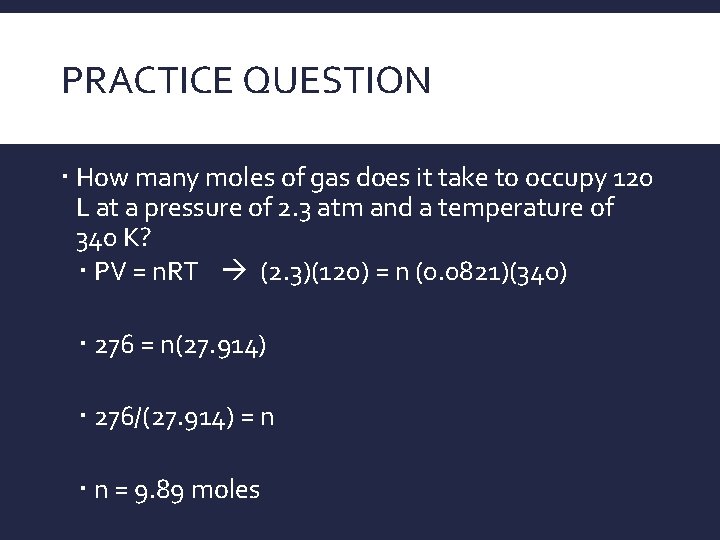
PRACTICE QUESTION How many moles of gas does it take to occupy 120 L at a pressure of 2. 3 atm and a temperature of 340 K? PV = n. RT (2. 3)(120) = n (0. 0821)(340) 276 = n(27. 914) 276/(27. 914) = n n = 9. 89 moles

GAS STOICHIOMETRY Moles Liters of a Gas STP - use 22. 4 L/mol Non-STP - use ideal gas law

PRACTICE QUESTION You are given 1 L of carbon monoxide at STP, how many moles of carbon monoxide do you have? 1 L x 1 mol = 0. 045 mol carbon monoxide 22. 4 L
 Buoyancyability
Buoyancyability Chapter 14 solids liquids and gases
Chapter 14 solids liquids and gases Kinetic molecular theory
Kinetic molecular theory Kinetic molecular theory volume
Kinetic molecular theory volume Adhesive force
Adhesive force Kinetic molecular theory of liquids
Kinetic molecular theory of liquids Kinetic energy molecular theory
Kinetic energy molecular theory Kinetic theory def
Kinetic theory def Timeline of kinetic molecular theory
Timeline of kinetic molecular theory Charles law in terms of kinetic molecular theory
Charles law in terms of kinetic molecular theory Postulates of kinetic theory of gas
Postulates of kinetic theory of gas Kinetic molecular theory
Kinetic molecular theory Write postulates of kinetic theory of gases
Write postulates of kinetic theory of gases Kinetic molecular theory formula
Kinetic molecular theory formula Kinetic molecular theory
Kinetic molecular theory Tenets of kinetic molecular theory
Tenets of kinetic molecular theory Properties of solids and liquids
Properties of solids and liquids Properties of solids liquids and gases with examples
Properties of solids liquids and gases with examples Kmt postulate
Kmt postulate Solid liquid gas particles
Solid liquid gas particles Gas particles are separated by relatively large distances
Gas particles are separated by relatively large distances Chapter 21: temperature, heat, and expansion answer key
Chapter 21: temperature, heat, and expansion answer key Gas like mixture of charged particles
Gas like mixture of charged particles Mot and vbt
Mot and vbt Molecular orbital theory vs valence bond theory
Molecular orbital theory vs valence bond theory Valence bond theory and molecular orbital theory
Valence bond theory and molecular orbital theory 11:55
11:55