Gas Laws Kinetic Theory Gases 1 Gas particles





- Slides: 9

Gas Laws

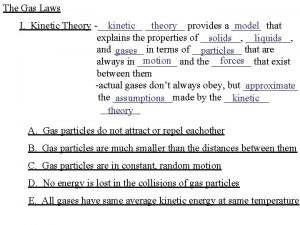
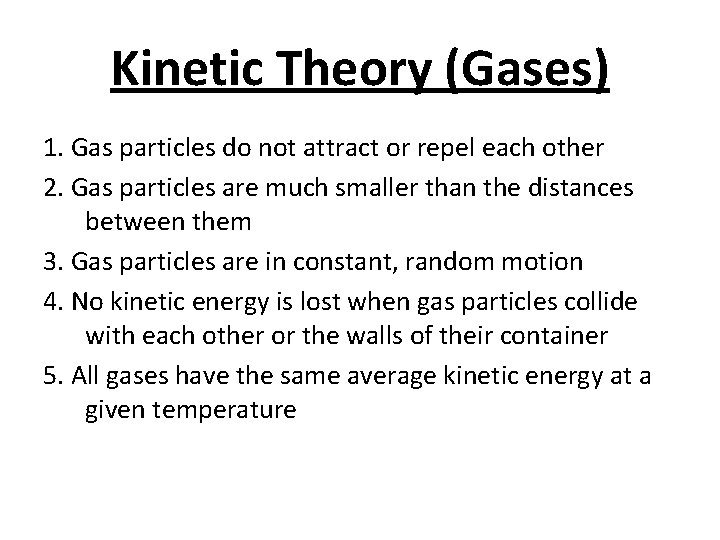
Kinetic Theory (Gases) 1. Gas particles do not attract or repel each other 2. Gas particles are much smaller than the distances between them 3. Gas particles are in constant, random motion 4. No kinetic energy is lost when gas particles collide with each other or the walls of their container 5. All gases have the same average kinetic energy at a given temperature

Properties of Gases Based on ideal gases and kinetic theory 1. FLUID – capable of flowing 2. No definite shape or volume * expand to fill container * takes the shape of the container 3. Transmit and exert pressure in all directions

Ideal Gases • describe the behavior of gases under “ideal” condition according to kinetic theory • includes a 4 th variable/factor affecting gases

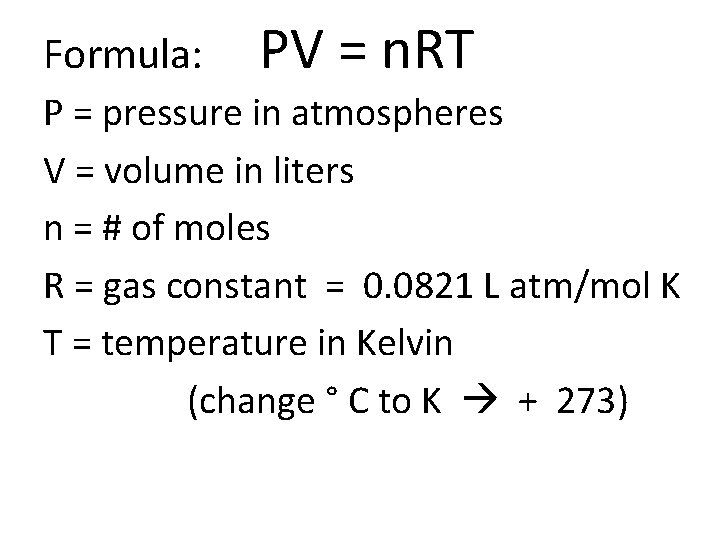
Formula: PV = n. RT P = pressure in atmospheres V = volume in liters n = # of moles R = gas constant = 0. 0821 L atm/mol K T = temperature in Kelvin (change ° C to K + 273)

EX: A sample of 11. 4 moles of dry ice (solid CO 2) becomes gas at room temperature (25 °C). Calculate the volume of CO 2 if pressure is 9. 62 atm.

EX: Average lung capacity of humans is 4000 m. L. Assuming you breathe pure oxygen, how many moles of O 2 gas can your lungs hold at 37°C (body temperature) and 827 mm pressure?

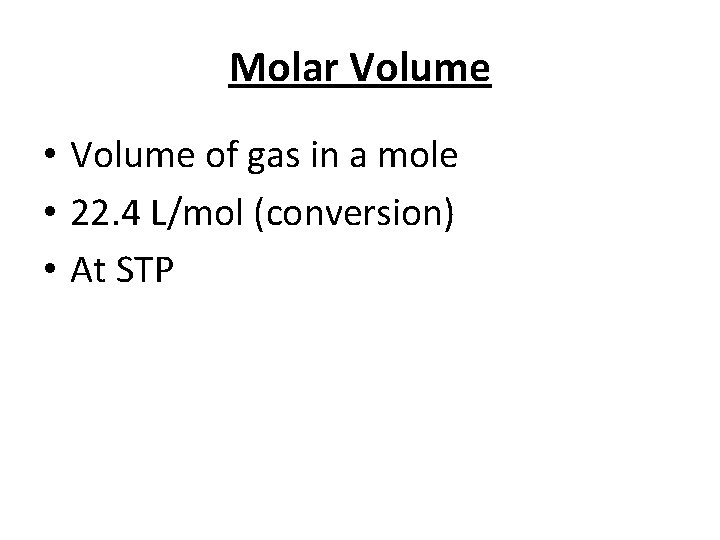
Molar Volume • Volume of gas in a mole • 22. 4 L/mol (conversion) • At STP

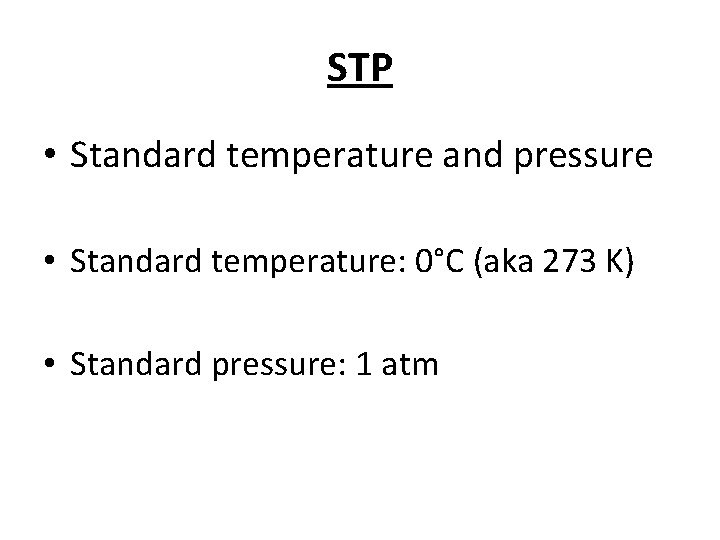
STP • Standard temperature and pressure • Standard temperature: 0°C (aka 273 K) • Standard pressure: 1 atm
 An explanation of how particles in matter behave
An explanation of how particles in matter behave Kinetic molecular theory
Kinetic molecular theory Kinetic particle model
Kinetic particle model Kinetic theory of gases
Kinetic theory of gases Three postulates of kinetic theory of gases
Three postulates of kinetic theory of gases Kinetic theory of gases
Kinetic theory of gases Write postulates of kinetic theory of gases
Write postulates of kinetic theory of gases Write the postulates of kinetic theory of gases
Write the postulates of kinetic theory of gases Kinetic theory
Kinetic theory Motion of particles in solids, liquids and gases
Motion of particles in solids, liquids and gases