States of Matter Kinetic Molecular Theory Explanation for







- Slides: 20

States of Matter

Kinetic Molecular Theory Explanation for atoms/molecules’ behavior within a state of matter. Views atoms/molecules within matter in constant motion. Explains properties associated with solids, liquids, and gases

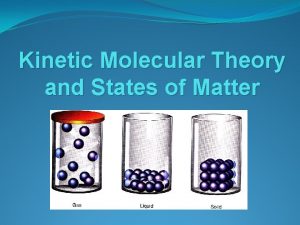
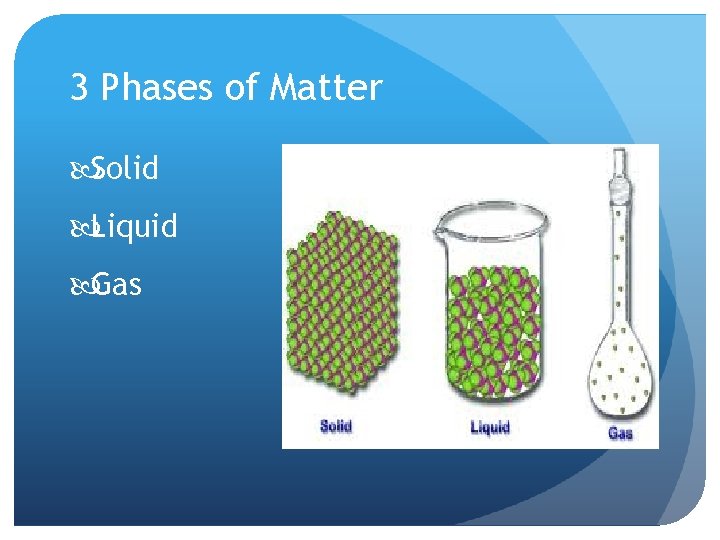
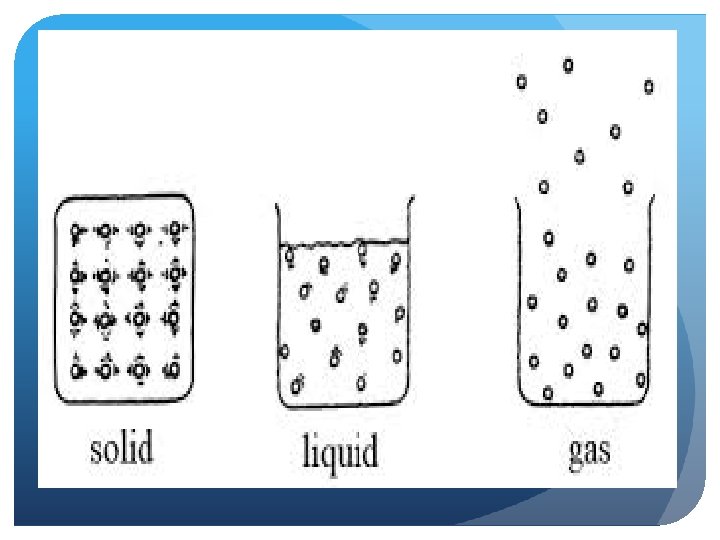
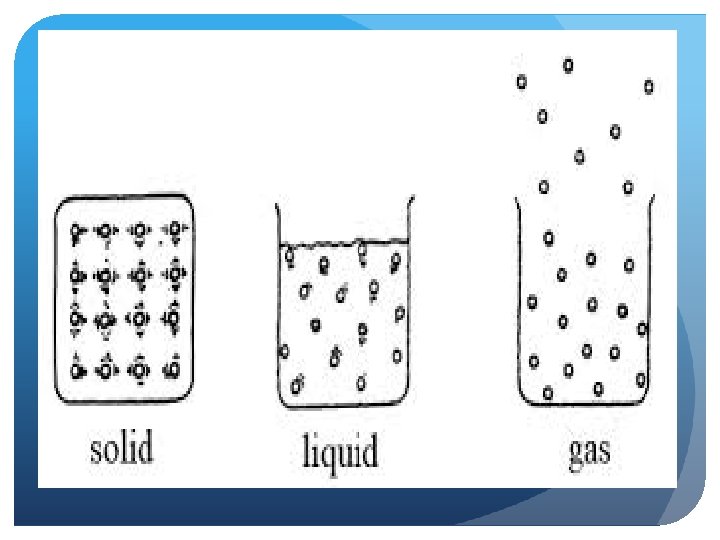
3 Phases of Matter Solid Liquid Gas

How do particles move in a solid?

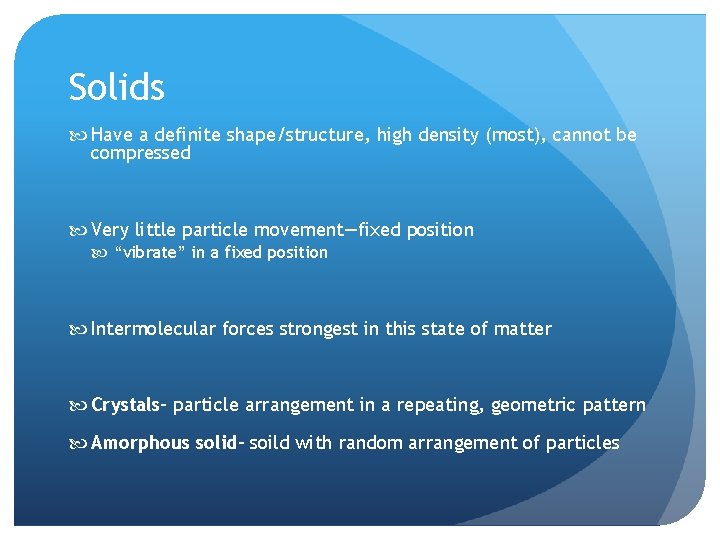
Solids Have a definite shape/structure, high density (most), cannot be compressed Very little particle movement—fixed position “vibrate” in a fixed position Intermolecular forces strongest in this state of matter Crystals– particle arrangement in a repeating, geometric pattern Amorphous solid– soild with random arrangement of particles



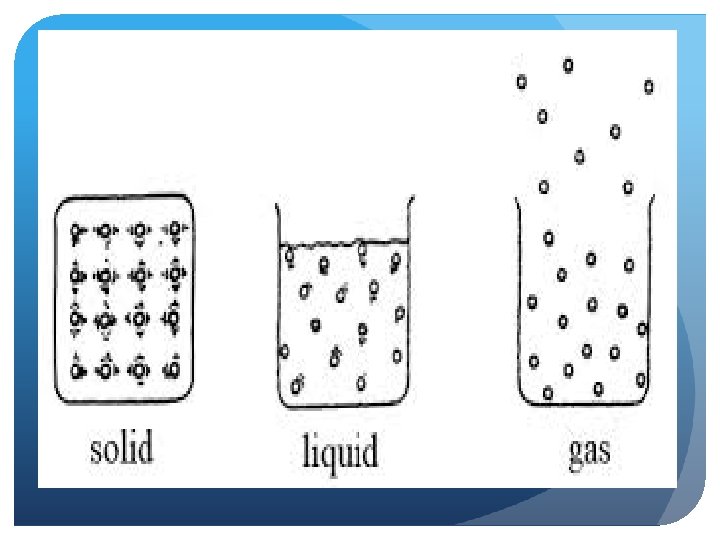
How do particles move in a liquid?

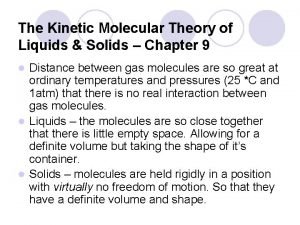
Liquids Limited structure to particles, definite volume Diffusion is possible (liquids can mix together), more compressible than solids Movement is less restricted, particles can move around and collide with each other Less intermolecular force attraction among these particles Conform to a container’s shape, exhibits “flow”—fluid Exhibit surface tension (force allowing the liquid to adhere to a surface)



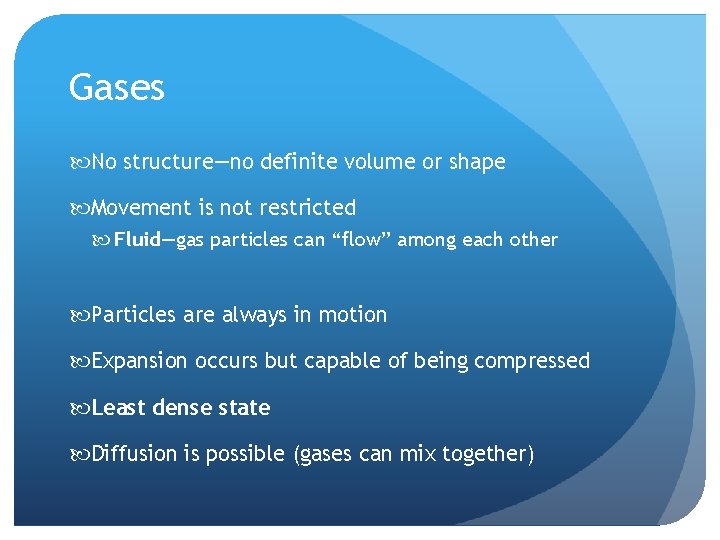
Gases No structure—no definite volume or shape Movement is not restricted Fluid—gas particles can “flow” among each other Particles are always in motion Expansion occurs but capable of being compressed Least dense state Diffusion is possible (gases can mix together)



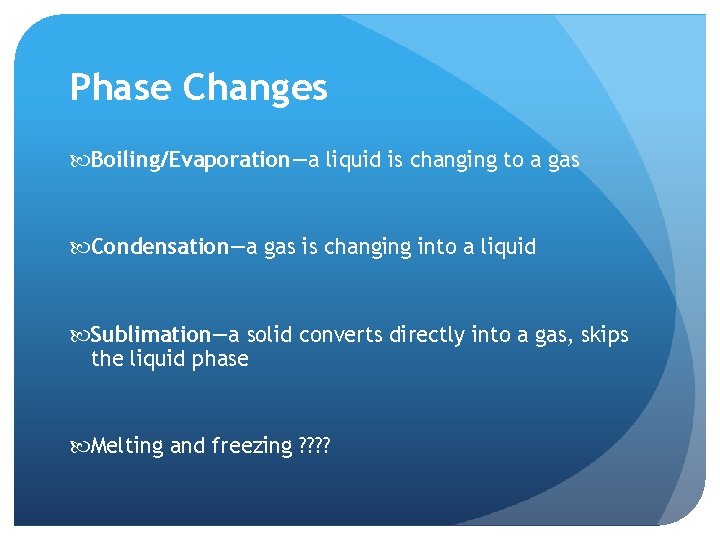
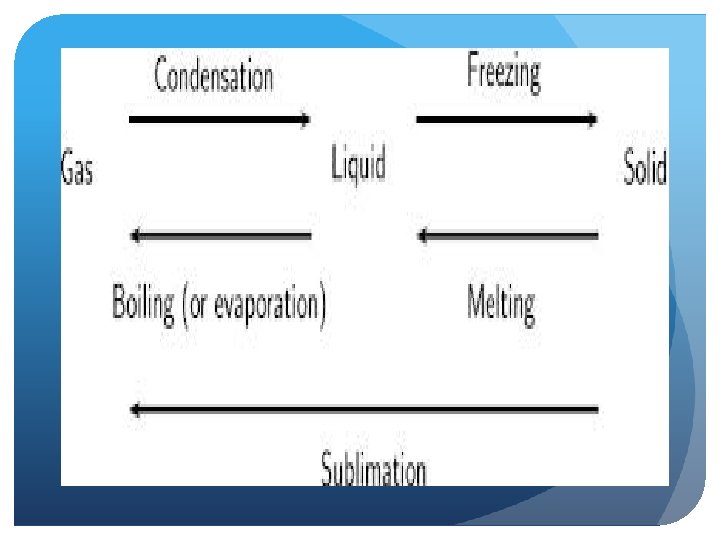
Phase Changes Boiling/Evaporation—a liquid is changing to a gas Condensation—a gas is changing into a liquid Sublimation—a solid converts directly into a gas, skips the liquid phase Melting and freezing ? ?


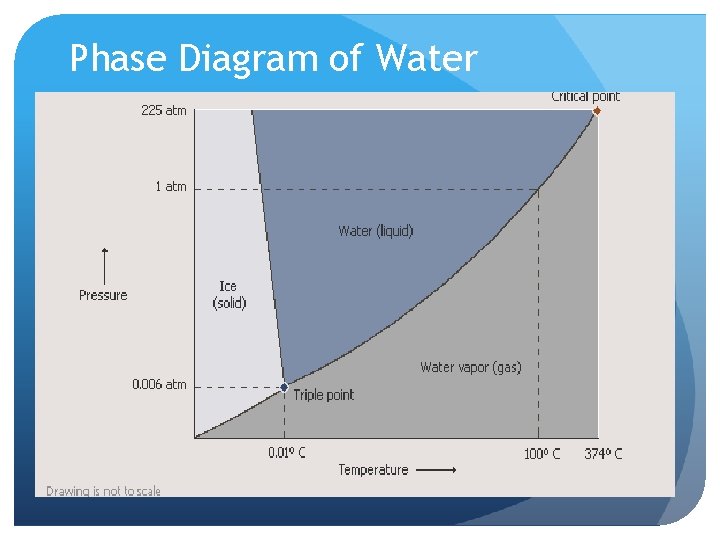
Phase Diagrams Graph plot of temperature vs. pressure Indicates what physical conditions MUST exist for each phase of a substance Specific for each substance

Phase Diagram of Water

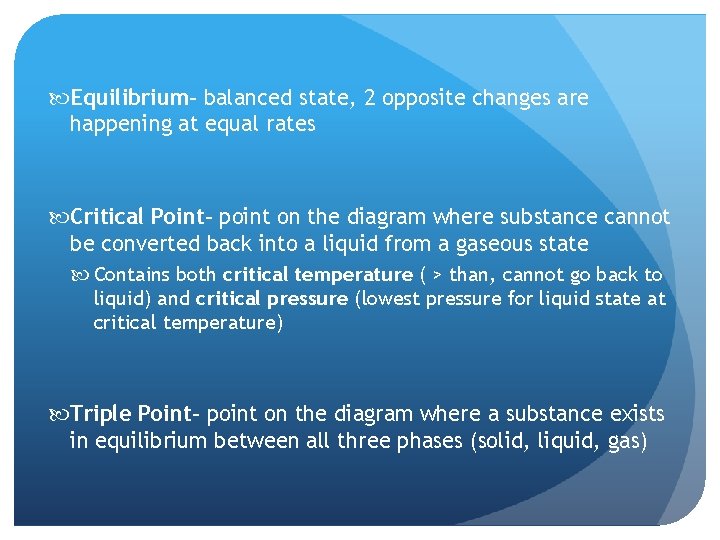
Equilibrium– balanced state, 2 opposite changes are happening at equal rates Critical Point– point on the diagram where substance cannot be converted back into a liquid from a gaseous state Contains both critical temperature ( > than, cannot go back to liquid) and critical pressure (lowest pressure for liquid state at critical temperature) Triple Point– point on the diagram where a substance exists in equilibrium between all three phases (solid, liquid, gas)

Melting Point– a substance goes from a solid to a liquid at this temperature Boiling Point– liquid vaporizes, the external atmosphere pressure = vapor pressure of the liquid
 The kinetic theory of matter states that
The kinetic theory of matter states that Kinetic molecular theory of solids
Kinetic molecular theory of solids Kinetic theory for ideal gases
Kinetic theory for ideal gases Kinetic molecular theory volume
Kinetic molecular theory volume Adhesive force
Adhesive force Kinetic molecular theory of liquids
Kinetic molecular theory of liquids Postulates of kinetic molecular theory
Postulates of kinetic molecular theory Kinetic molecular theory def
Kinetic molecular theory def Timeline of kinetic molecular theory
Timeline of kinetic molecular theory Charles law in terms of kinetic molecular theory
Charles law in terms of kinetic molecular theory Postulates of kinetic molecular theory
Postulates of kinetic molecular theory Kenitic molecular theory
Kenitic molecular theory Write the postulates of kinetic theory of gases
Write the postulates of kinetic theory of gases Kinetic molecular theory formula
Kinetic molecular theory formula Kinetic molecular theory
Kinetic molecular theory Tenets of kinetic molecular theory
Tenets of kinetic molecular theory Kinetic theory of matter definition
Kinetic theory of matter definition Kinetic theory of matter
Kinetic theory of matter The kinetic theory explains how particles in matter behave
The kinetic theory explains how particles in matter behave Particle theory freezing
Particle theory freezing What is a kinetic theory of matter
What is a kinetic theory of matter