Gas Laws WHS AP Chemistry Spring 2014 Elements

- Slides: 32

Gas Laws WHS AP Chemistry Spring 2014

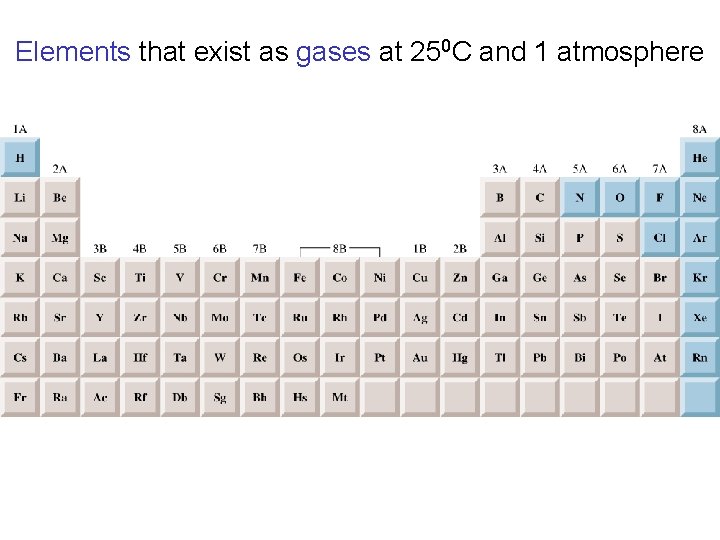
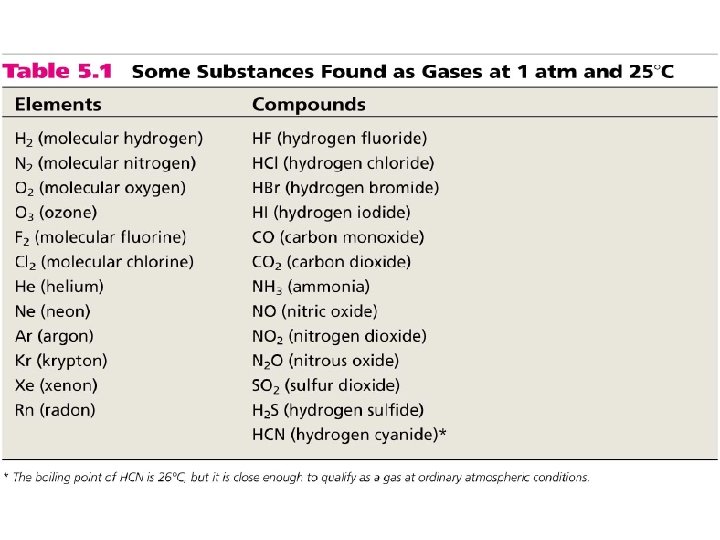
Elements that exist as gases at 250 C and 1 atmosphere


Physical Characteristics of Gases • Gases assume the volume and shape of their containers. • Gases are the most compressible state of matter. • Gases will mix evenly and completely when confined to the same container. • Gases have much lower densities than liquids and solids.

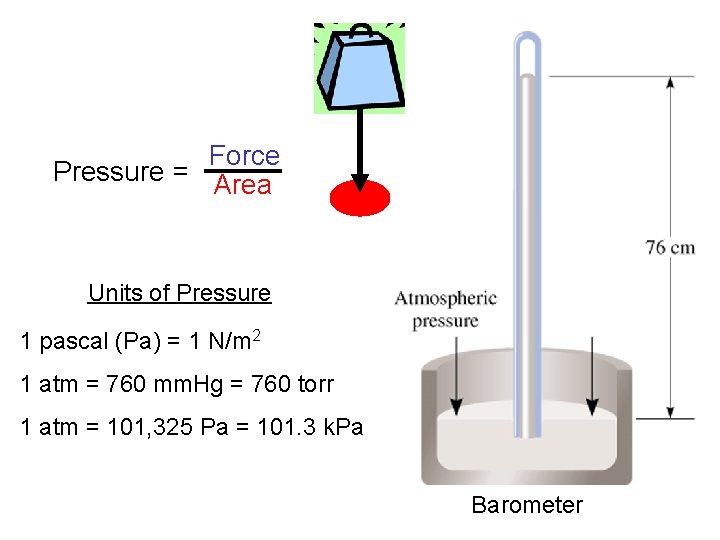
Force Pressure = Area Units of Pressure 1 pascal (Pa) = 1 N/m 2 1 atm = 760 mm. Hg = 760 torr 1 atm = 101, 325 Pa = 101. 3 k. Pa Barometer

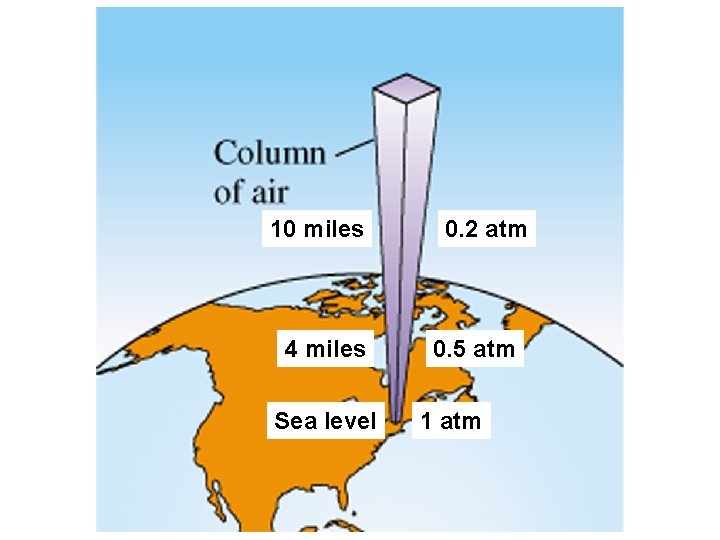
10 miles 4 miles Sea level 0. 2 atm 0. 5 atm 1 atm


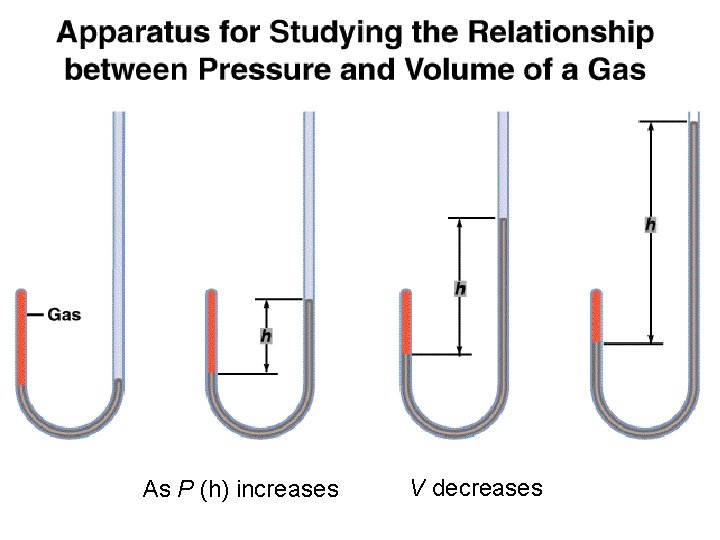
As P (h) increases V decreases

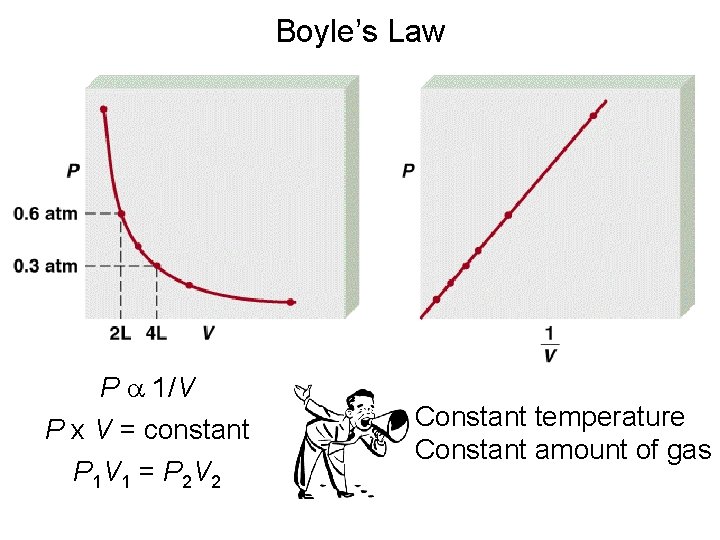
Boyle’s Law P a 1/V P x V = constant P 1 V 1 = P 2 V 2 Constant temperature Constant amount of gas

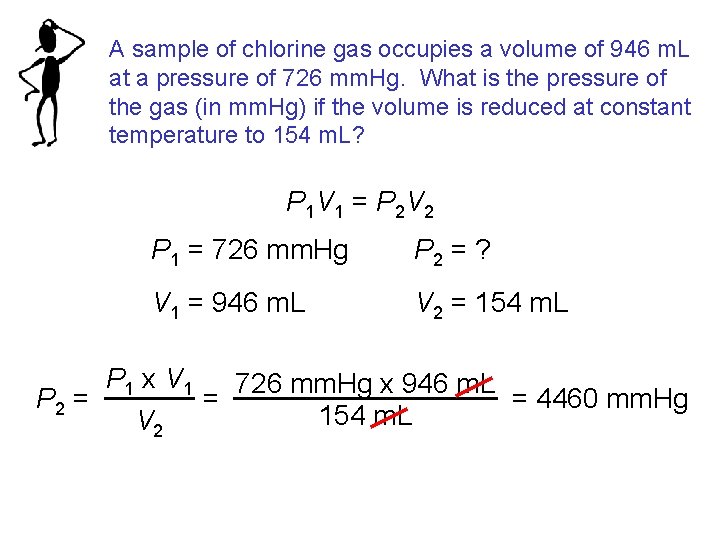
A sample of chlorine gas occupies a volume of 946 m. L at a pressure of 726 mm. Hg. What is the pressure of the gas (in mm. Hg) if the volume is reduced at constant temperature to 154 m. L? P 1 V 1 = P 2 V 2 P 1 = 726 mm. Hg P 2 = ? V 1 = 946 m. L V 2 = 154 m. L P 1 x V 1 726 mm. Hg x 946 m. L = P 2 = = 4460 mm. Hg 154 m. L V 2

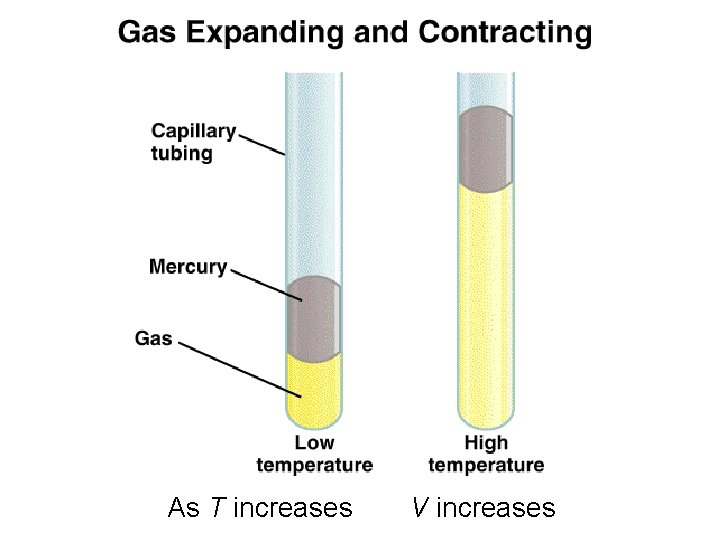
As T increases V increases

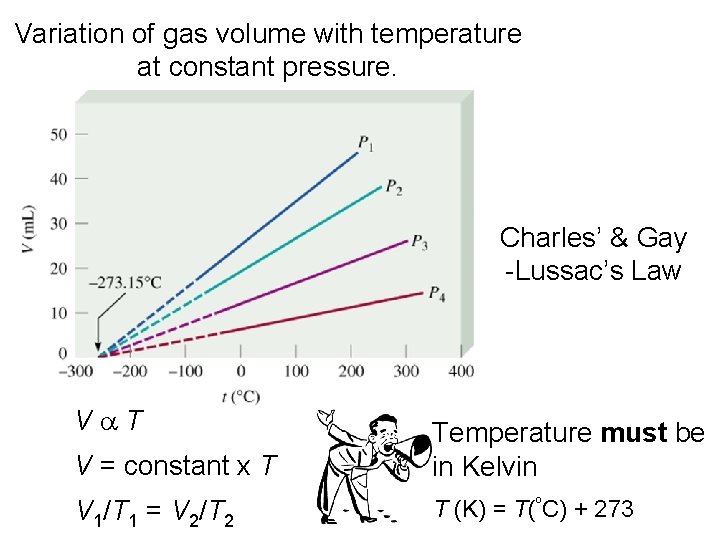
Variation of gas volume with temperature at constant pressure. Charles’ & Gay -Lussac’s Law Va. T V = constant x T Temperature must be in Kelvin V 1/T 1 = V 2/T 2 T (K) = T(ºC) + 273

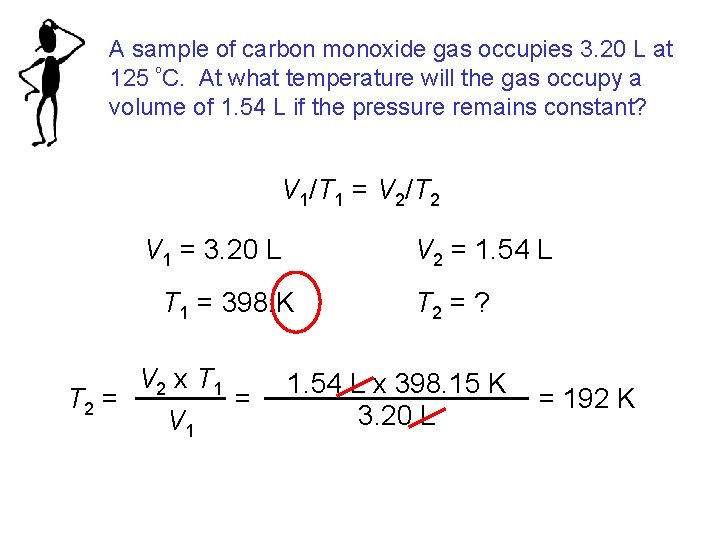
A sample of carbon monoxide gas occupies 3. 20 L at 125 ºC. At what temperature will the gas occupy a volume of 1. 54 L if the pressure remains constant? V 1/T 1 = V 2/T 2 V 1 = 3. 20 L V 2 = 1. 54 L T 1 = 398. K V 2 x T 1 = T 2 = V 1 T 2 = ? 1. 54 L x 398. 15 K 3. 20 L = 192 K

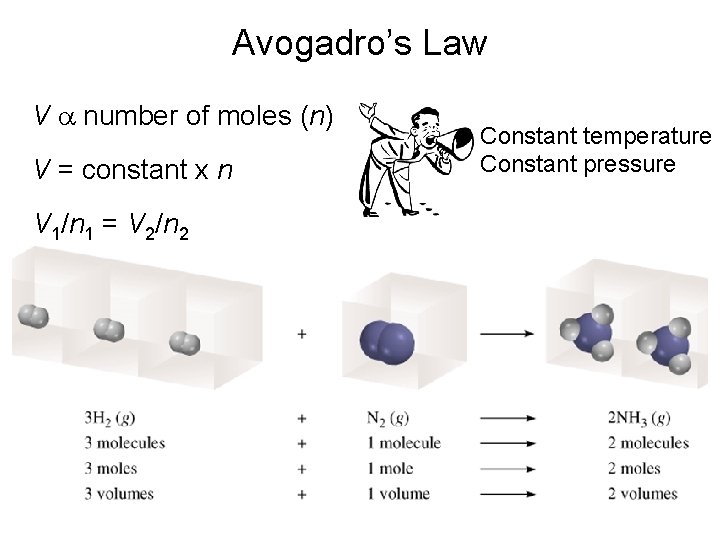
Avogadro’s Law V a number of moles (n) V = constant x n V 1/n 1 = V 2/n 2 Constant temperature Constant pressure

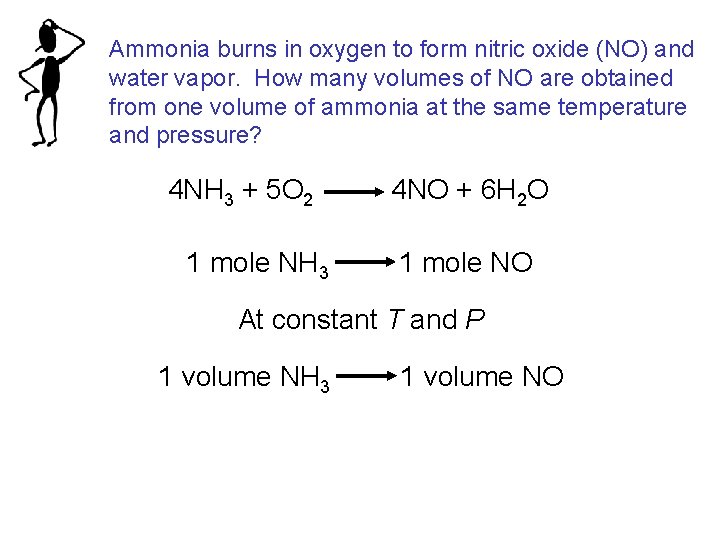
Ammonia burns in oxygen to form nitric oxide (NO) and water vapor. How many volumes of NO are obtained from one volume of ammonia at the same temperature and pressure? 4 NH 3 + 5 O 2 1 mole NH 3 4 NO + 6 H 2 O 1 mole NO At constant T and P 1 volume NH 3 1 volume NO

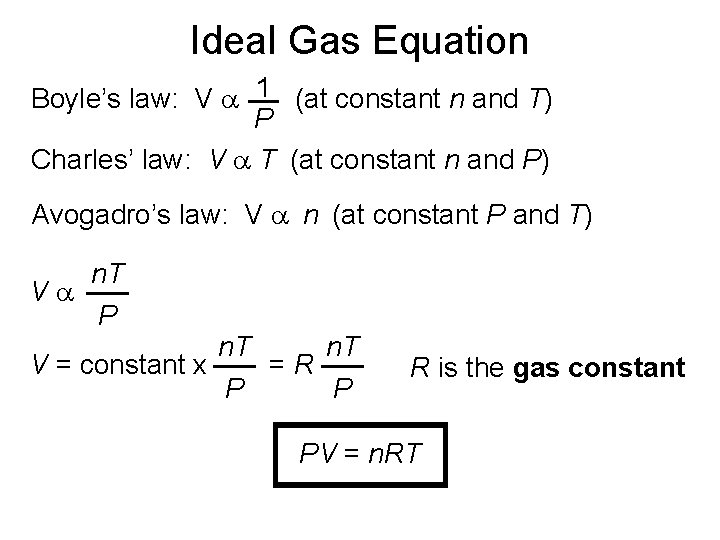
Ideal Gas Equation Boyle’s law: V a 1 (at constant n and T) P Charles’ law: V a T (at constant n and P) Avogadro’s law: V a n (at constant P and T) Va n. T P n. T V = constant x =R P P R is the gas constant PV = n. RT

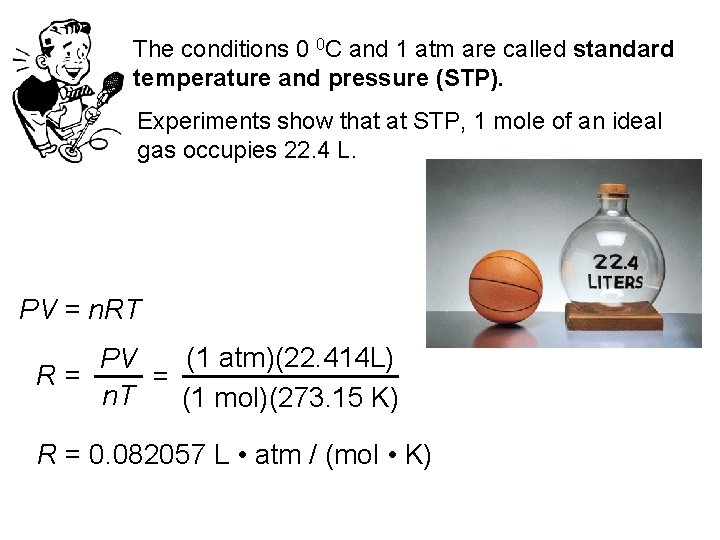
The conditions 0 0 C and 1 atm are called standard temperature and pressure (STP). Experiments show that at STP, 1 mole of an ideal gas occupies 22. 4 L. PV = n. RT (1 atm)(22. 414 L) PV R= = n. T (1 mol)(273. 15 K) R = 0. 082057 L • atm / (mol • K)

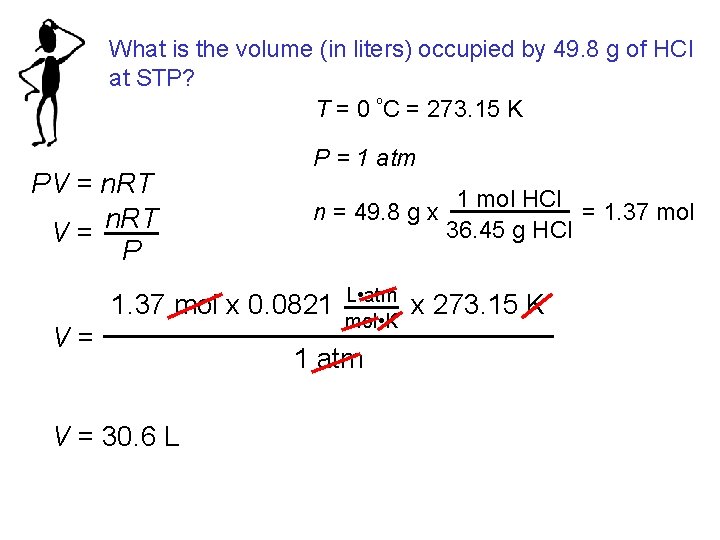
What is the volume (in liters) occupied by 49. 8 g of HCl at STP? T = 0 ºC = 273. 15 K PV = n. RT V= P P = 1 atm n = 49. 8 g x 1. 37 mol x 0. 0821 V= V = 30. 6 L L • atm mol • K 1 atm 1 mol HCl = 1. 37 mol 36. 45 g HCl x 273. 15 K

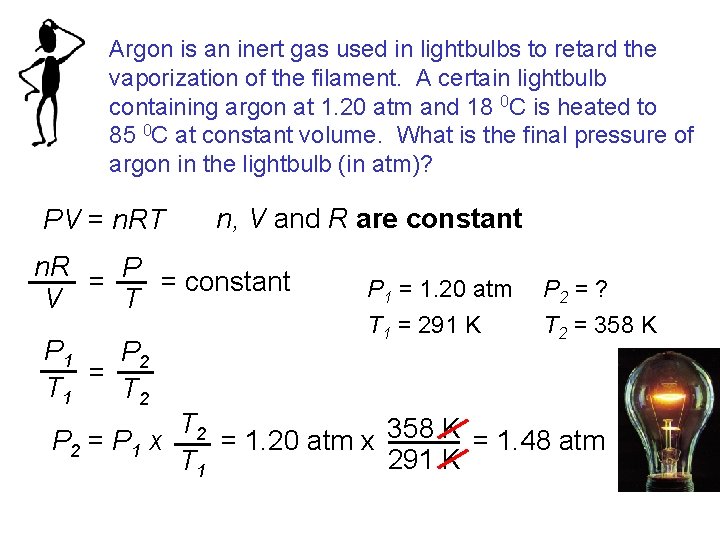
Argon is an inert gas used in lightbulbs to retard the vaporization of the filament. A certain lightbulb containing argon at 1. 20 atm and 18 0 C is heated to 85 0 C at constant volume. What is the final pressure of argon in the lightbulb (in atm)? PV = n. RT n, V and R are constant n. R = P = constant T V P 1 P 2 = T 1 T 2 P 1 = 1. 20 atm T 1 = 291 K P 2 = ? T 2 = 358 K T 2 = 1. 20 atm x 358 K = 1. 48 atm P 2 = P 1 x 291 K T 1

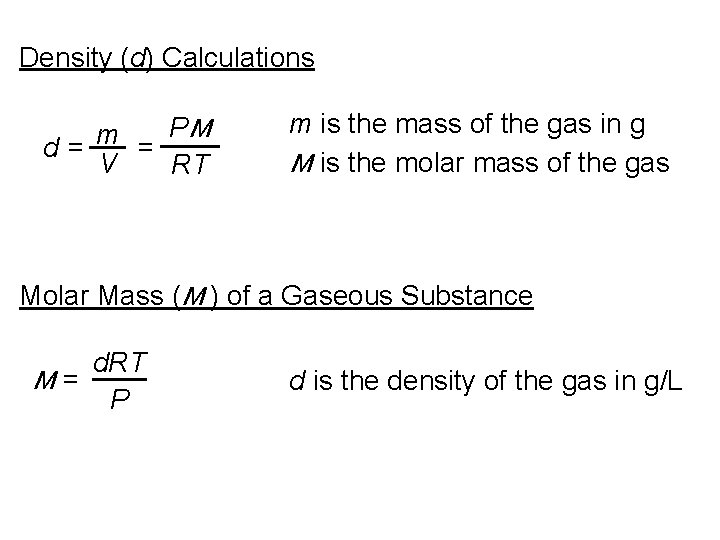
Density (d) Calculations PM m d= = V RT m is the mass of the gas in g M is the molar mass of the gas Molar Mass (M ) of a Gaseous Substance d. RT M= P d is the density of the gas in g/L

Gas Stoichiometry What is the volume of CO 2 produced at 370 C and 1. 00 atm when 5. 60 g of glucose are used up in the reaction: C 6 H 12 O 6 (s) + 6 O 2 (g) 6 CO 2 (g) + 6 H 2 O (l) g C 6 H 12 O 6 mol C 6 H 12 O 6 5. 60 g C 6 H 12 O 6 x 6 mol CO 2 1 mol C 6 H 12 O 6 x = 0. 187 mol CO 2 180 g C 6 H 12 O 6 1 mol C 6 H 12 O 6 V= n. RT = P mol CO 2 V CO 2 L • atm x 310. 15 K mol • K 1. 00 atm 0. 187 mol x 0. 0821 = 4. 76 L

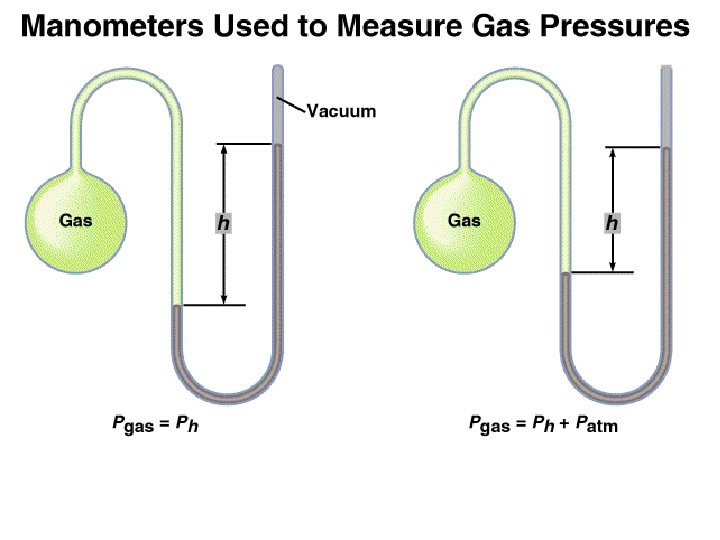
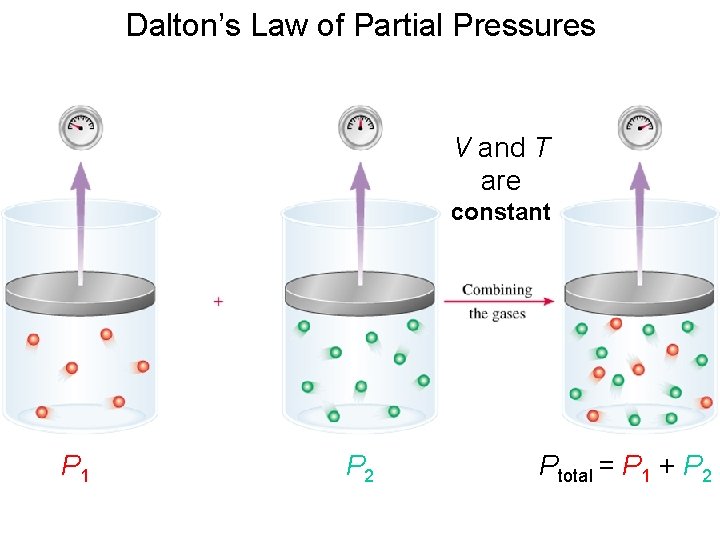
Dalton’s Law of Partial Pressures V and T are constant P 1 P 2 Ptotal = P 1 + P 2

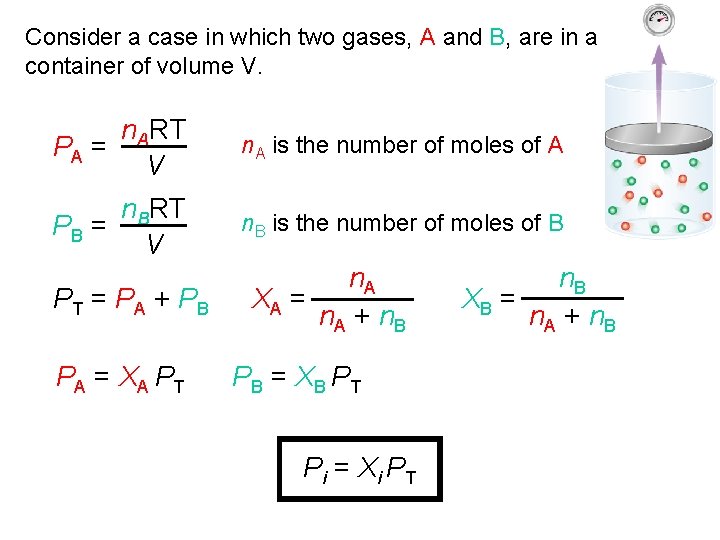
Consider a case in which two gases, A and B, are in a container of volume V. n. ART PA = V n. A is the number of moles of A n. BRT PB = V n. B is the number of moles of B PT = PA + PB PA = XA PT n. A XA = n. A + n. B PB = XB PT Pi = Xi PT n. B XB = n. A + n. B

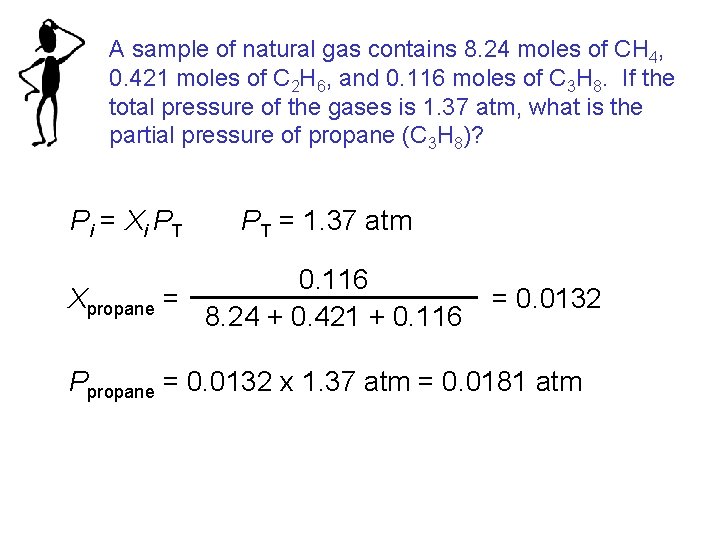
A sample of natural gas contains 8. 24 moles of CH 4, 0. 421 moles of C 2 H 6, and 0. 116 moles of C 3 H 8. If the total pressure of the gases is 1. 37 atm, what is the partial pressure of propane (C 3 H 8)? Pi = Xi PT PT = 1. 37 atm 0. 116 Xpropane = 8. 24 + 0. 421 + 0. 116 = 0. 0132 Ppropane = 0. 0132 x 1. 37 atm = 0. 0181 atm

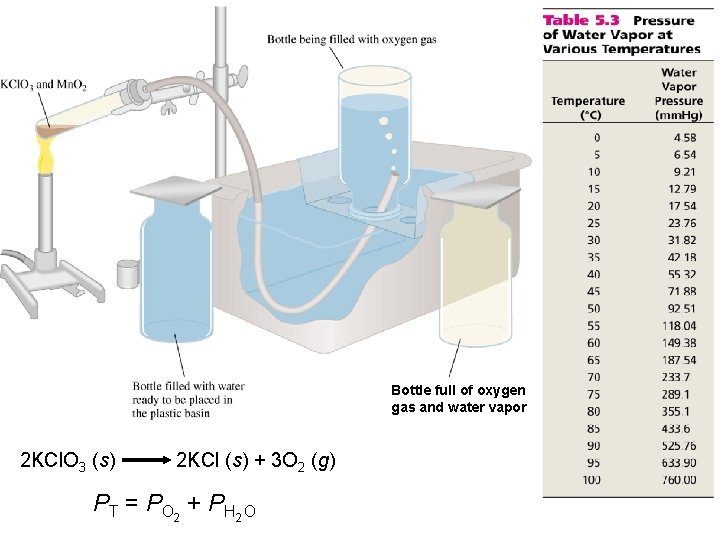
Bottle full of oxygen gas and water vapor 2 KCl. O 3 (s) 2 KCl (s) + 3 O 2 (g) PT = PO 2 + PH 2 O

Kinetic Molecular Theory of Gases 1. A gas is composed of molecules that are separated from each other by distances far greater than their own dimensions. The molecules can be considered to be points; that is, they possess mass but have negligible volume. 2. Gas molecules are in constant motion in random directions. Collisions among molecules are perfectly elastic. 3. Gas molecules exert neither attractive nor repulsive forces on one another. 4. The average kinetic energy of the molecules is proportional to the temperature of the gas in Kelvins. Any two gases at the same temperature will have the same average kinetic energy.

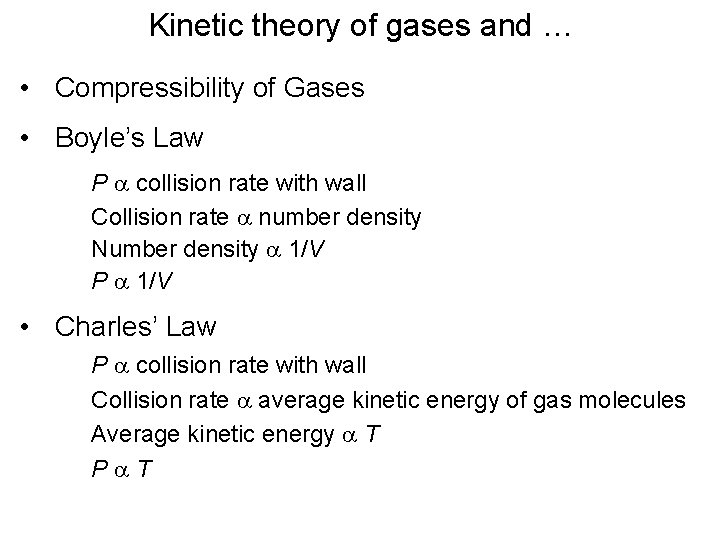
Kinetic theory of gases and … • Compressibility of Gases • Boyle’s Law P a collision rate with wall Collision rate a number density Number density a 1/V P a 1/V • Charles’ Law P a collision rate with wall Collision rate a average kinetic energy of gas molecules Average kinetic energy a T Pa. T

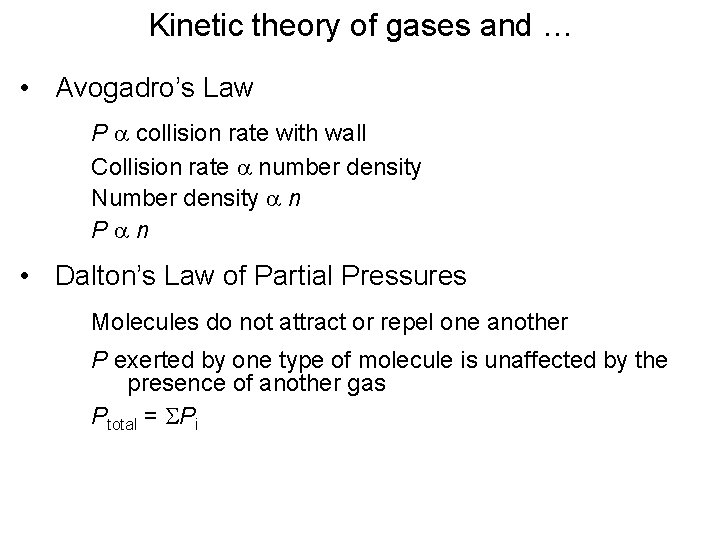
Kinetic theory of gases and … • Avogadro’s Law P a collision rate with wall Collision rate a number density Number density a n Pan • Dalton’s Law of Partial Pressures Molecules do not attract or repel one another P exerted by one type of molecule is unaffected by the presence of another gas Ptotal = SPi

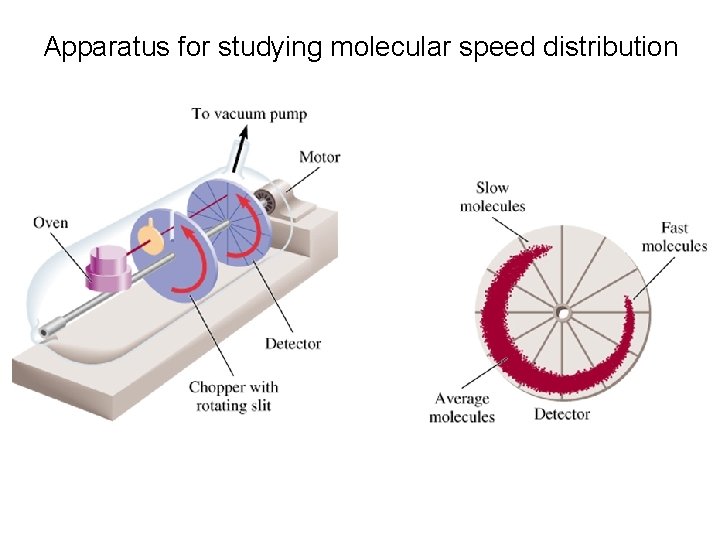
Apparatus for studying molecular speed distribution

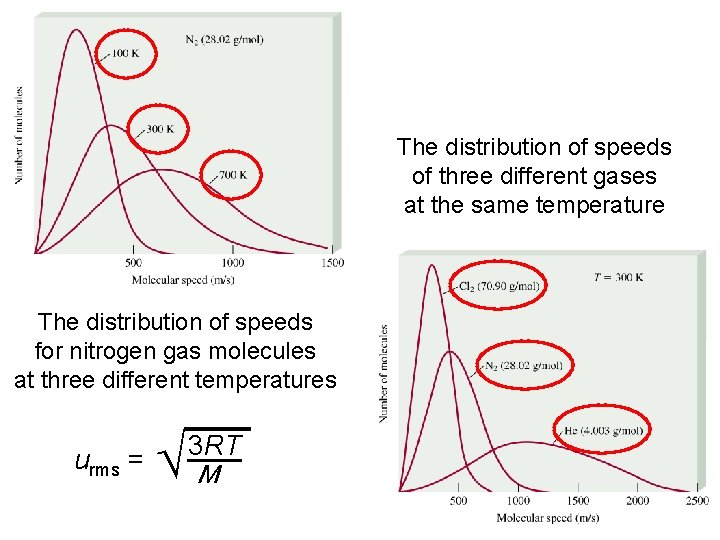
The distribution of speeds of three different gases at the same temperature The distribution of speeds for nitrogen gas molecules at three different temperatures urms = M 3 RT

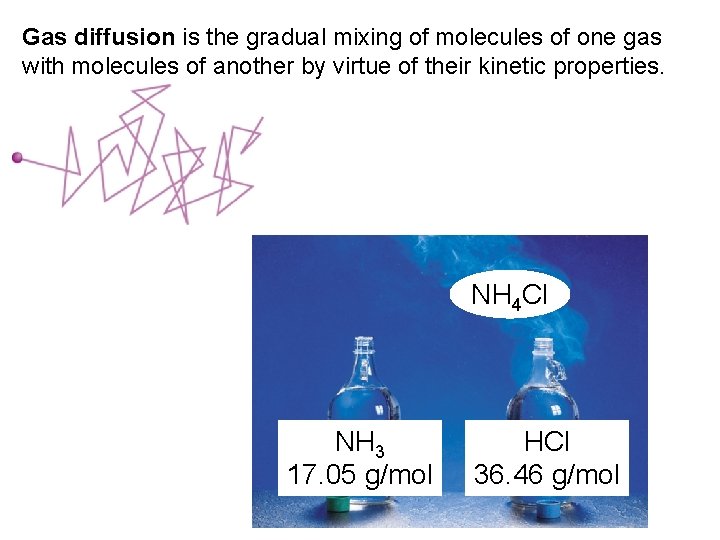
Gas diffusion is the gradual mixing of molecules of one gas with molecules of another by virtue of their kinetic properties. NH 4 Cl NH 3 17. 05 g/mol HCl 36. 46 g/mol

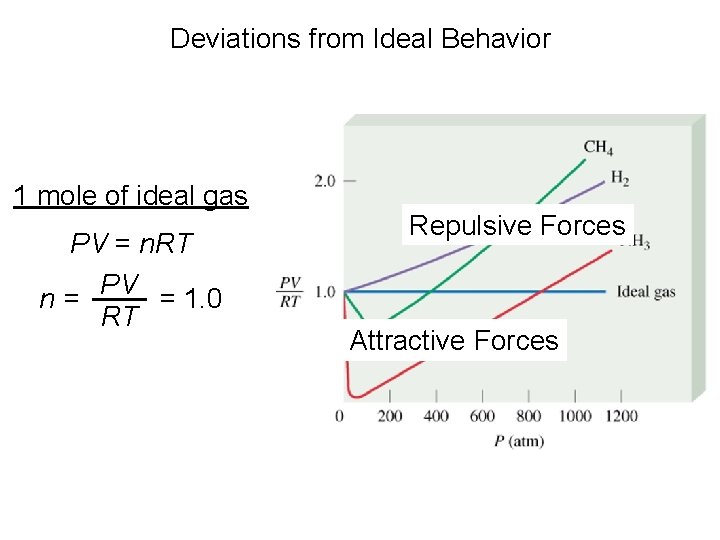
Deviations from Ideal Behavior 1 mole of ideal gas PV = n. RT PV = 1. 0 n= RT Repulsive Forces Attractive Forces