Discussion 8 2 The Ideal Gas Law 1

![Think About It!!! • List the variable(s) [P, V, T, # of moles] that Think About It!!! • List the variable(s) [P, V, T, # of moles] that](https://slidetodoc.com/presentation_image/6e0726b39363a151033e551ee1f48e14/image-2.jpg)


- Slides: 8

Discussion 8 -2 The Ideal Gas Law 1. How is an ideal gas different from a real gas? 2. What is the Ideal Gas Law? 3. How is the Ideal Gas Law used to solve problems? A. Conditions staying the same B. Conditions changing
![Think About It List the variables P V T of moles that Think About It!!! • List the variable(s) [P, V, T, # of moles] that](https://slidetodoc.com/presentation_image/6e0726b39363a151033e551ee1f48e14/image-2.jpg)
Think About It!!! • List the variable(s) [P, V, T, # of moles] that are changing for the underlined item in these scenarios. 1. A scuba diver puts on his scuba tank and jumps into the freezing Arctic waters where he dives to a depth of 500 m. (Assume that he is not using the air in his tank yet!) 2. A unopened bag of potato chips is left in the car on a hot day.

1. How is an ideal gas different from a real gas? • Ideal Gases • Real Gases • When does a real gas behave most like an ideal gas?

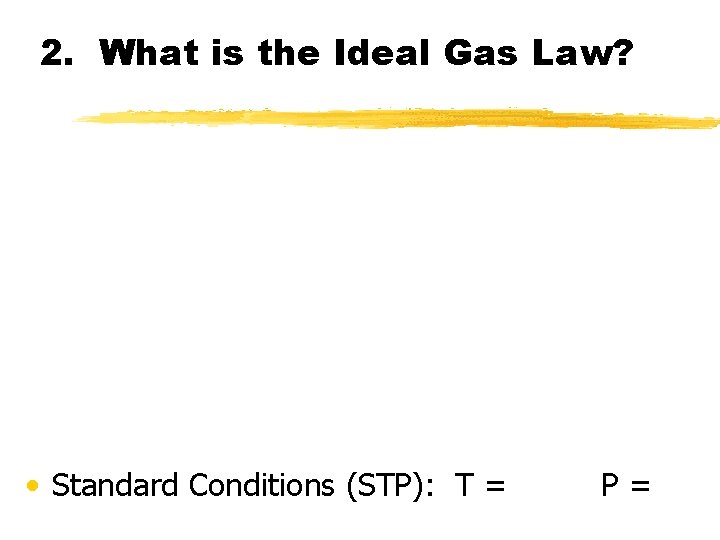
2. What is the Ideal Gas Law? • Standard Conditions (STP): T = P=

3. How is the Ideal Gas Law used to solve problems? Steps to Solve ALL Ideal Gas Law Problems 1. Underline what you are given, and circle what you are asked to find. 2. Ask yourself whether the conditions are staying the same or changing. A. If the conditions are staying the same, use PV=n. RT B. If the conditions are changing, use P 1 V 1 = n. RT 1 P 2 V 2 = n. RT 2 --Cross-out any variables that are not changing. Re-write the equation. 3. Check to make sure all units are in L, atm, mol, and K. Convert if necessary. 4. SOLVE!

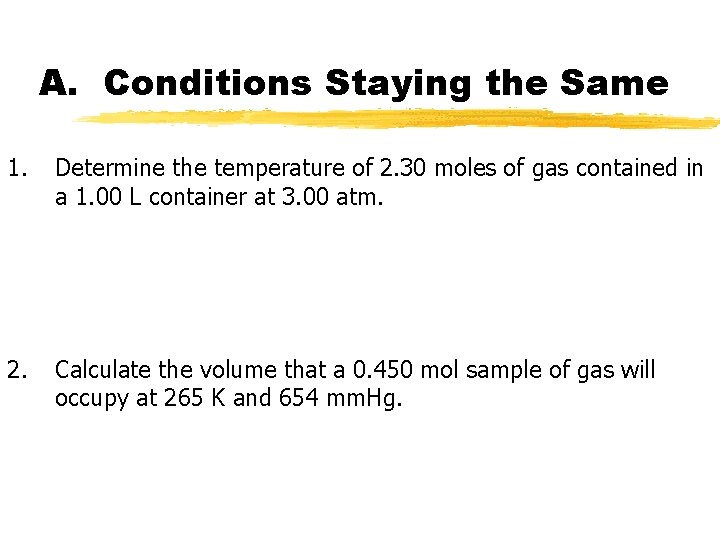
A. Conditions Staying the Same 1. Determine the temperature of 2. 30 moles of gas contained in a 1. 00 L container at 3. 00 atm. 2. Calculate the volume that a 0. 450 mol sample of gas will occupy at 265 K and 654 mm. Hg.

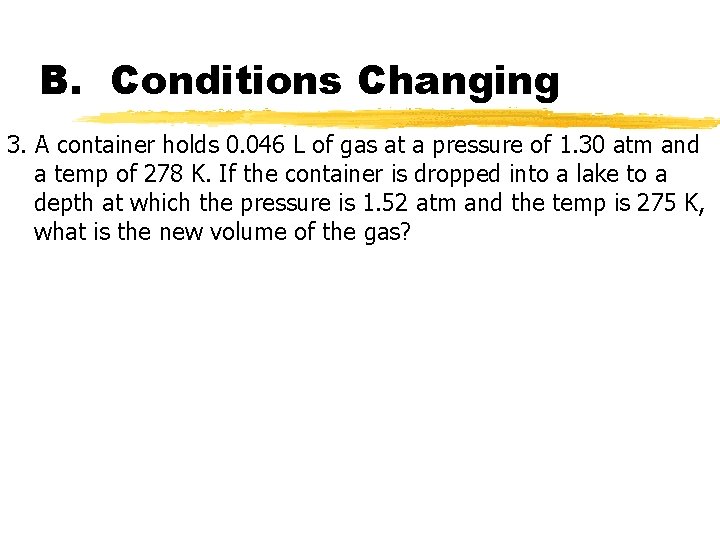
B. Conditions Changing 3. A container holds 0. 046 L of gas at a pressure of 1. 30 atm and a temp of 278 K. If the container is dropped into a lake to a depth at which the pressure is 1. 52 atm and the temp is 275 K, what is the new volume of the gas?

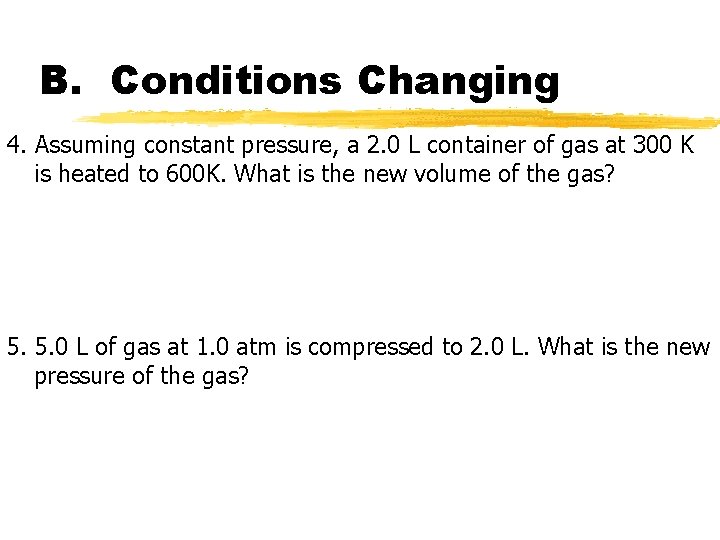
B. Conditions Changing 4. Assuming constant pressure, a 2. 0 L container of gas at 300 K is heated to 600 K. What is the new volume of the gas? 5. 5. 0 L of gas at 1. 0 atm is compressed to 2. 0 L. What is the new pressure of the gas?