Gases Kinetic Theory of Ideal Gas Gas Laws





- Slides: 35

Gases Kinetic Theory of Ideal Gas, Gas Laws & Equation Combined Gas Laws, Numerical value of R

The Unique Gas Phase Physical properties of a gas are nearly independent of its chemical identity! Gas behavior is markedly different than solid or liquid behavior and have lower densities than the liquid and solids. They assume the volume and shape of their containers. They are the most compressible state of matters Gases will mix evenly and completely when confined to the same container. Pressure is simply a force exerted over a surface area.

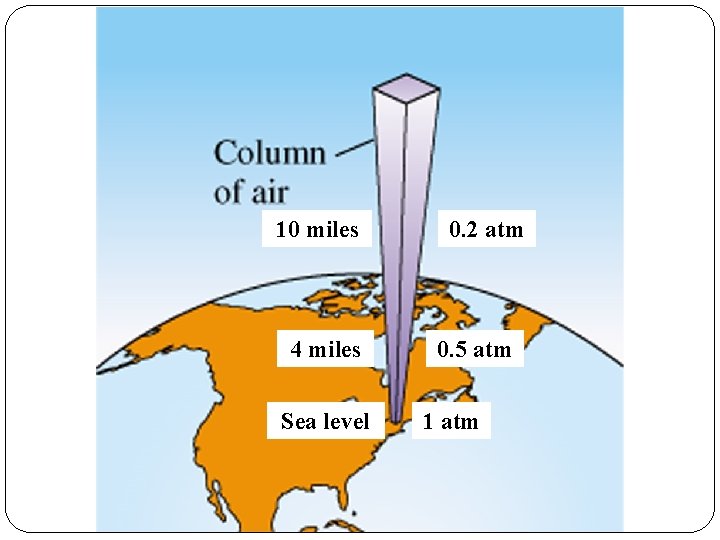
10 miles 4 miles Sea level 0. 2 atm 0. 5 atm 1 atm

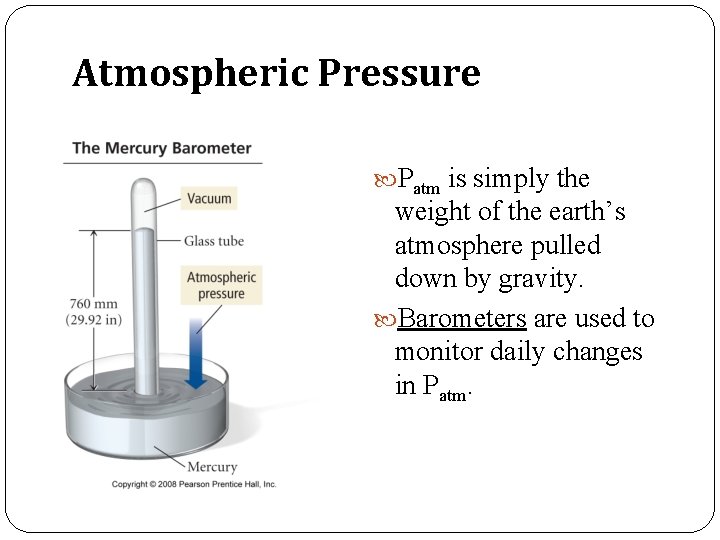
Atmospheric Pressure Patm is simply the weight of the earth’s atmosphere pulled down by gravity. Barometers are used to monitor daily changes in Patm.

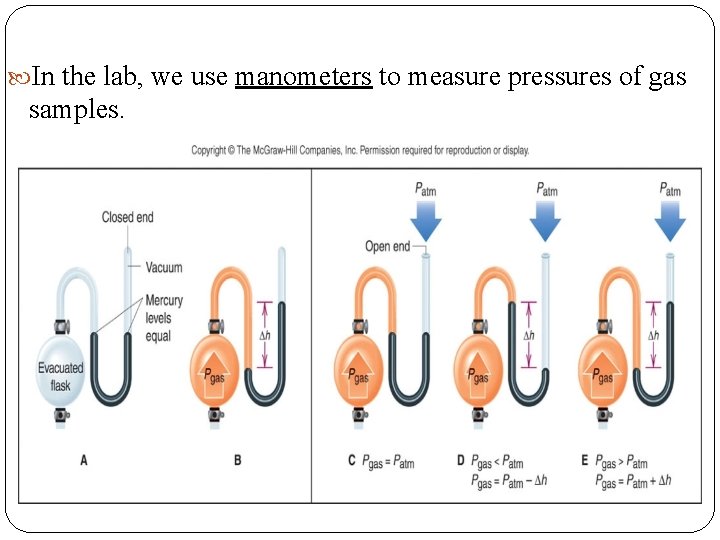
In the lab, we use manometers to measure pressures of gas samples.

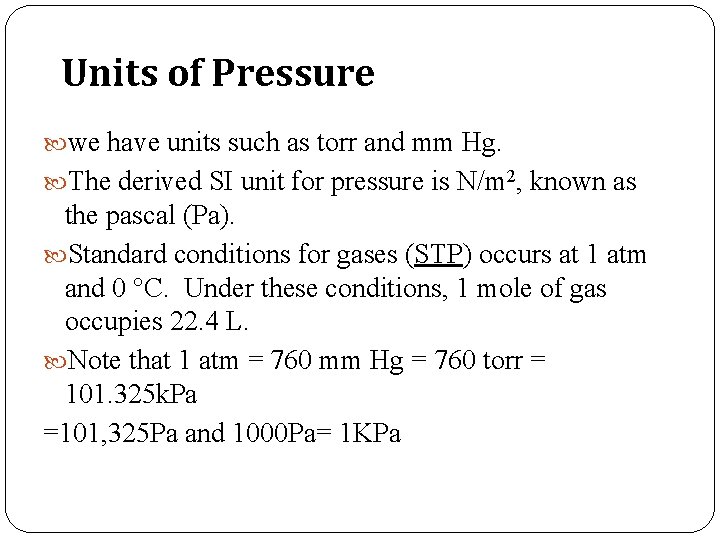
Units of Pressure we have units such as torr and mm Hg. The derived SI unit for pressure is N/m 2, known as the pascal (Pa). Standard conditions for gases (STP) occurs at 1 atm and 0 °C. Under these conditions, 1 mole of gas occupies 22. 4 L. Note that 1 atm = 760 mm Hg = 760 torr = 101. 325 k. Pa =101, 325 Pa and 1000 Pa= 1 KPa

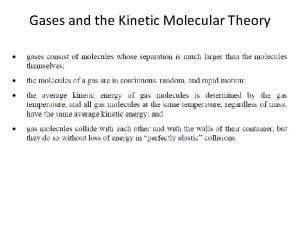
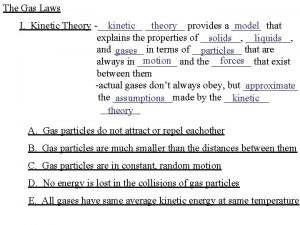
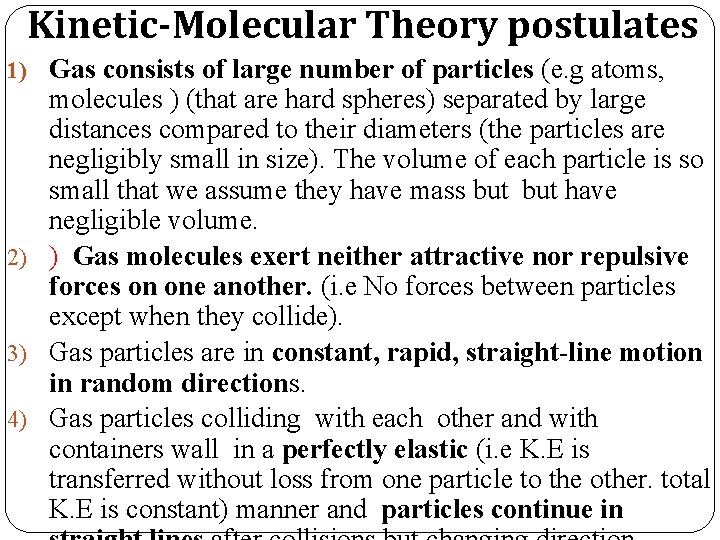
Kinetic-Molecular Theory postulates 1) Gas consists of large number of particles (e. g atoms, molecules ) (that are hard spheres) separated by large distances compared to their diameters (the particles are negligibly small in size). The volume of each particle is so small that we assume they have mass but have negligible volume. 2) ) Gas molecules exert neither attractive nor repulsive forces on one another. (i. e No forces between particles except when they collide). 3) Gas particles are in constant, rapid, straight-line motion in random directions. 4) Gas particles colliding with each other and with containers wall in a perfectly elastic (i. e K. E is transferred without loss from one particle to the other. total K. E is constant) manner and particles continue in

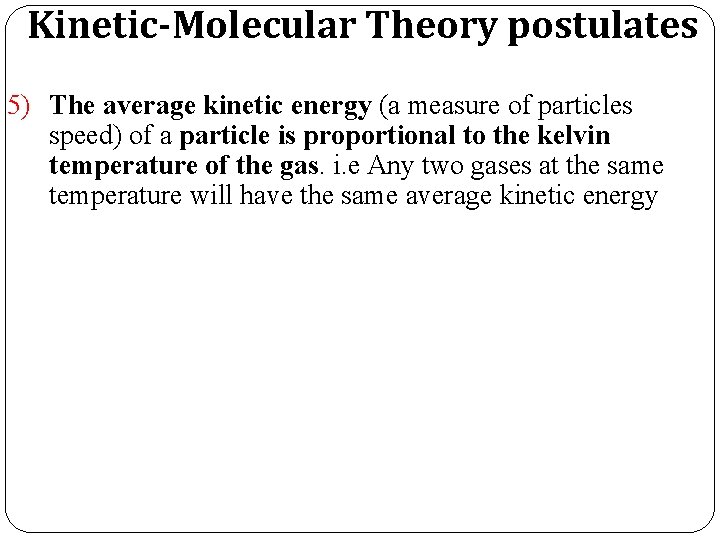
Kinetic-Molecular Theory postulates 5) The average kinetic energy (a measure of particles speed) of a particle is proportional to the kelvin temperature of the gas. i. e Any two gases at the same temperature will have the same average kinetic energy

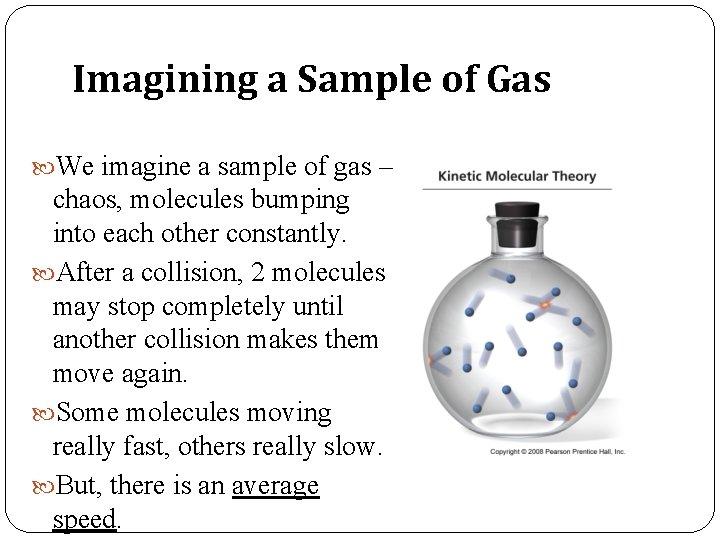
Imagining a Sample of Gas We imagine a sample of gas – chaos, molecules bumping into each other constantly. After a collision, 2 molecules may stop completely until another collision makes them move again. Some molecules moving really fast, others really slow. But, there is an average speed.

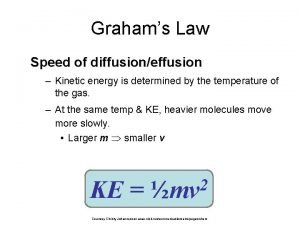
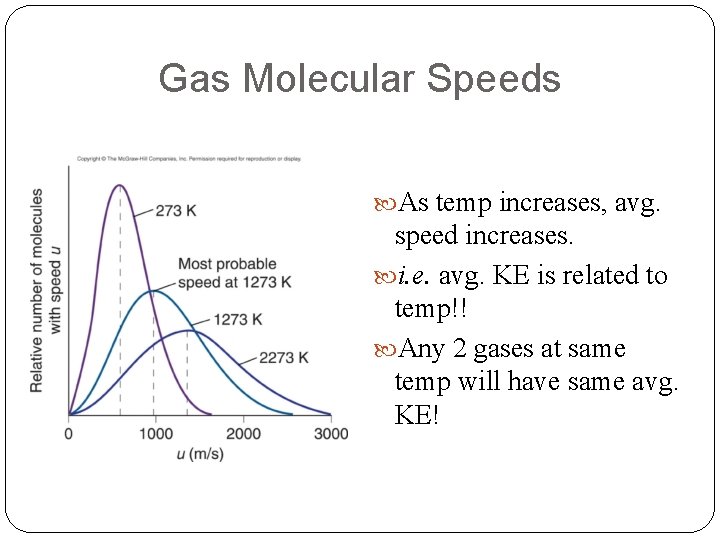
Gas Molecular Speeds As temp increases, avg. speed increases. i. e. avg. KE is related to temp!! Any 2 gases at same temp will have same avg. KE!

Why Do Gas Laws Work So Well? Recall that the gas laws apply to any gas – the chemical identity is not important. Gas particles only interact when they collide. Since this interaction is so short, chemical properties don’t have time to take effect!!

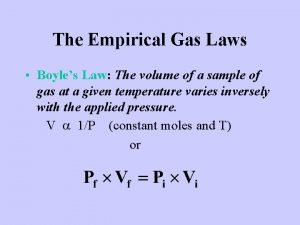
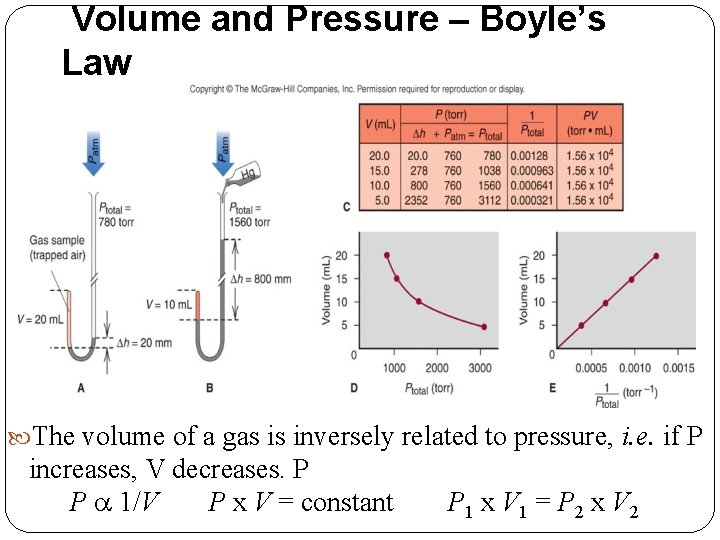
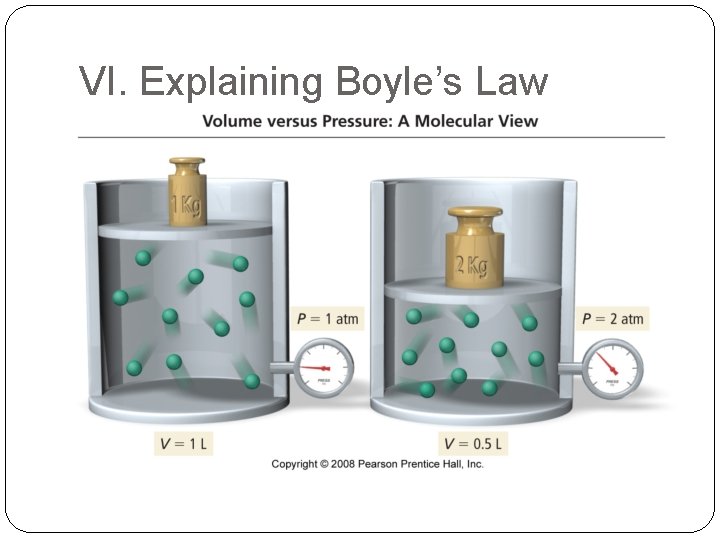
Volume and Pressure – Boyle’s Law The volume of a gas is inversely related to pressure, i. e. if P increases, V decreases. P P a 1/V P x V = constant P 1 x V 1 = P 2 x V 2

VI. Explaining Boyle’s Law

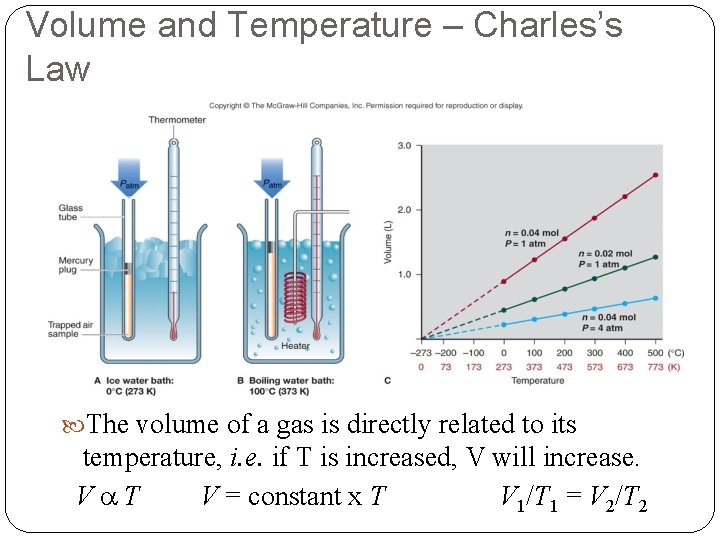
Volume and Temperature – Charles’s Law The volume of a gas is directly related to its temperature, i. e. if T is increased, V will increase. Va. T V = constant x T V 1/T 1 = V 2/T 2

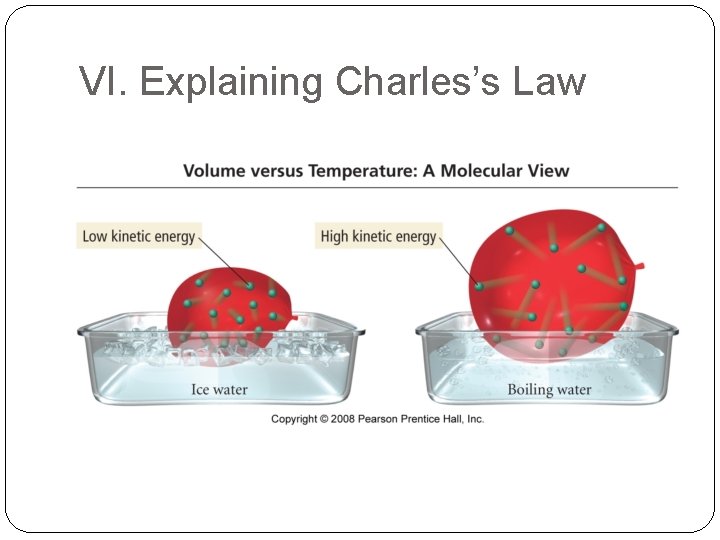
VI. Explaining Charles’s Law

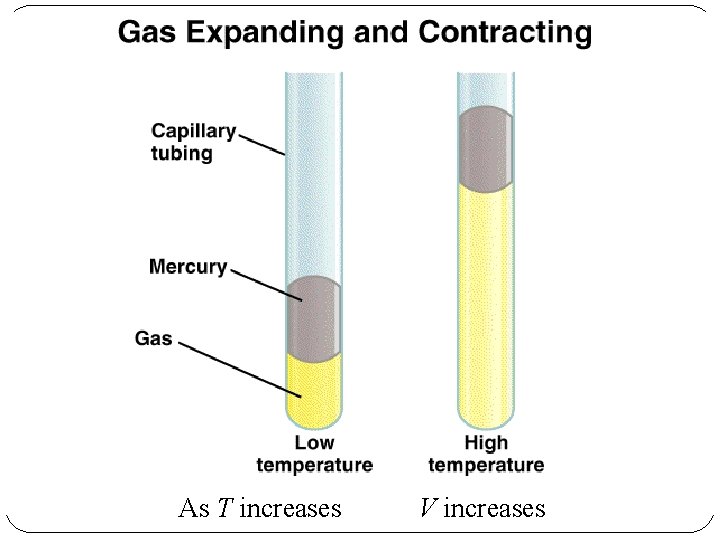
As T increases V increases

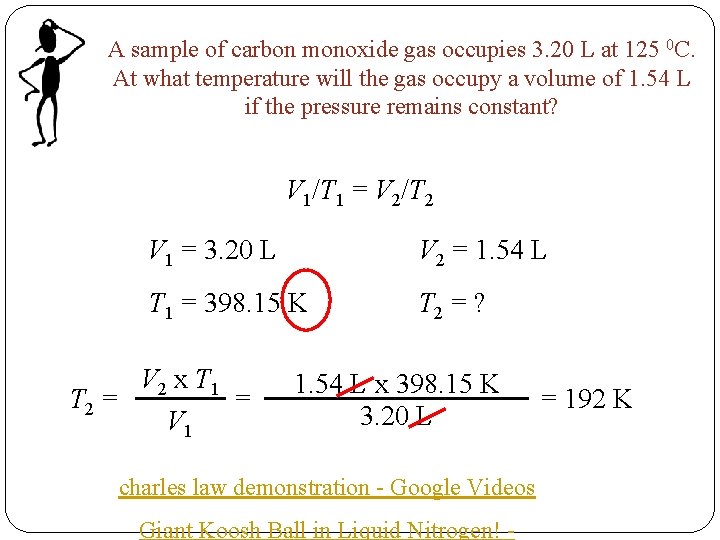
A sample of carbon monoxide gas occupies 3. 20 L at 125 0 C. At what temperature will the gas occupy a volume of 1. 54 L if the pressure remains constant? V 1/T 1 = V 2/T 2 V 1 = 3. 20 L V 2 = 1. 54 L T 1 = 398. 15 K T 2 = ? V 2 x T 1 = T 2 = V 1 1. 54 L x 398. 15 K 3. 20 L charles law demonstration - Google Videos Giant Koosh Ball in Liquid Nitrogen! - = 192 K

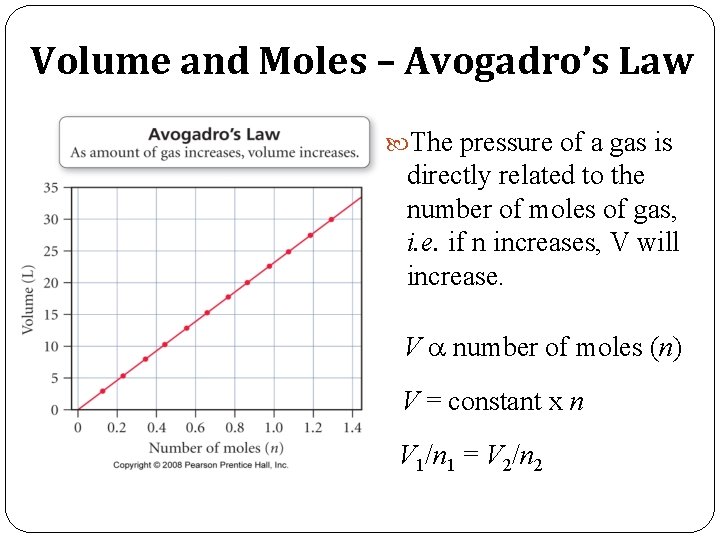
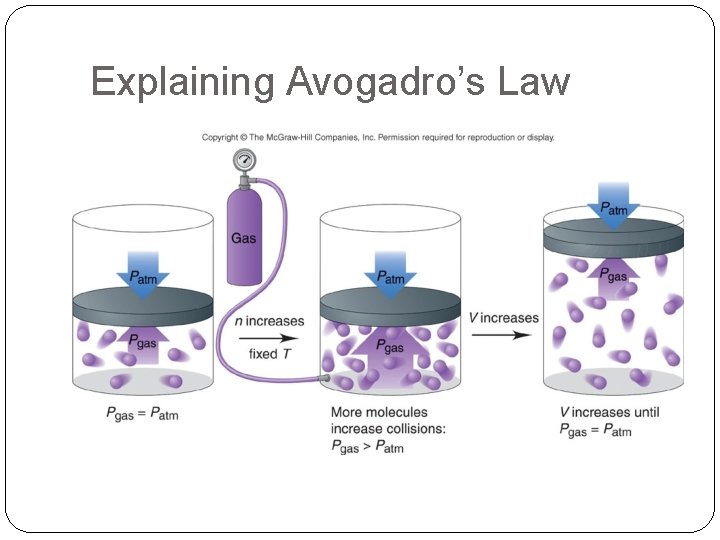
Volume and Moles – Avogadro’s Law The pressure of a gas is directly related to the number of moles of gas, i. e. if n increases, V will increase. V a number of moles (n) V = constant x n V 1/n 1 = V 2/n 2

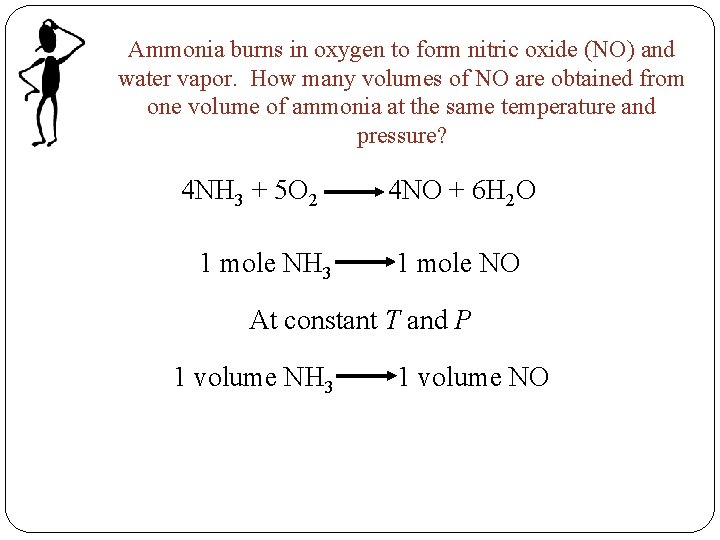
Ammonia burns in oxygen to form nitric oxide (NO) and water vapor. How many volumes of NO are obtained from one volume of ammonia at the same temperature and pressure? 4 NH 3 + 5 O 2 1 mole NH 3 4 NO + 6 H 2 O 1 mole NO At constant T and P 1 volume NH 3 1 volume NO

Explaining Avogadro’s Law

Mixtures of Gases Dalton's law of partial pressure states: the total pressure of a mixture of gases is equal to the sum of the partial pressures of the component gases.

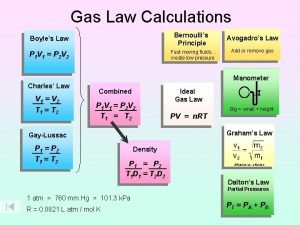
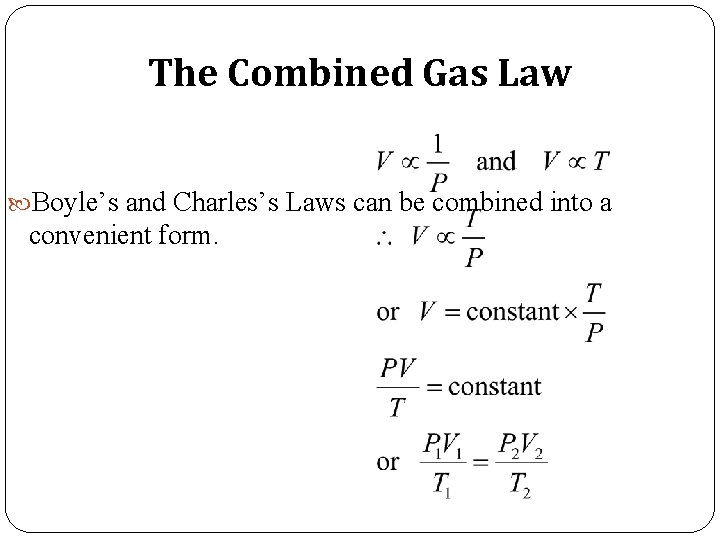
The Combined Gas Law Boyle’s and Charles’s Laws can be combined into a convenient form.

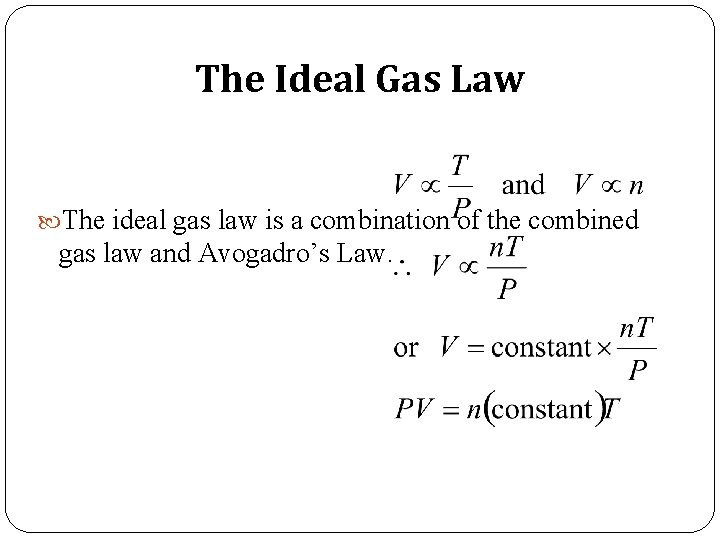
The Ideal Gas Law The ideal gas law is a combination of the combined gas law and Avogadro’s Law. R = 0. 082058 L atm/K mole

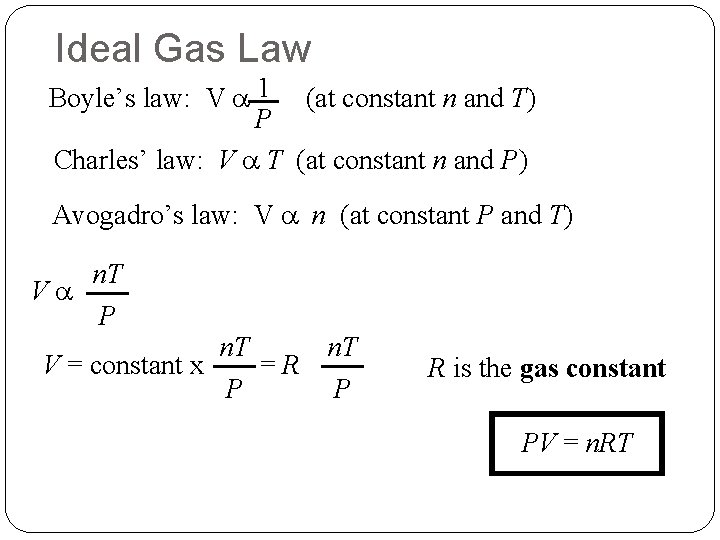
Ideal Gas Law Boyle’s law: V a 1 (at constant n and T) P Charles’ law: V a T (at constant n and P) Avogadro’s law: V a n (at constant P and T) Va n. T P n. T V = constant x =R P P R is the gas constant PV = n. RT

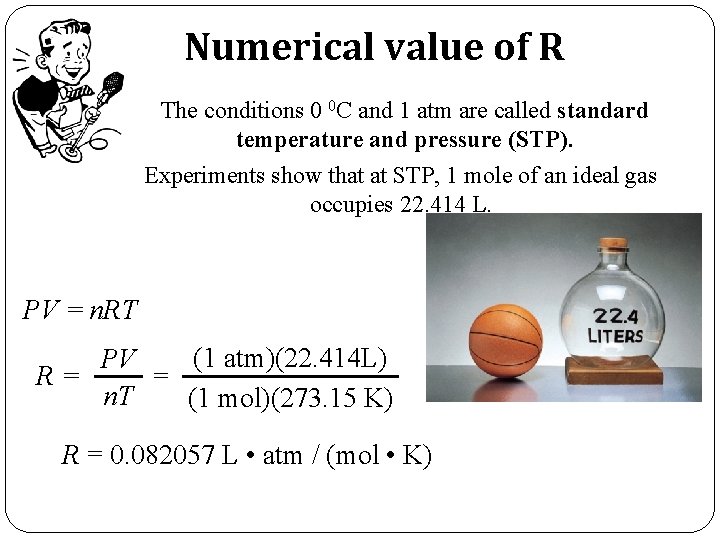
Numerical value of R The conditions 0 0 C and 1 atm are called standard temperature and pressure (STP). Experiments show that at STP, 1 mole of an ideal gas occupies 22. 414 L. PV = n. RT (1 atm)(22. 414 L) PV R= = n. T (1 mol)(273. 15 K) R = 0. 082057 L • atm / (mol • K)

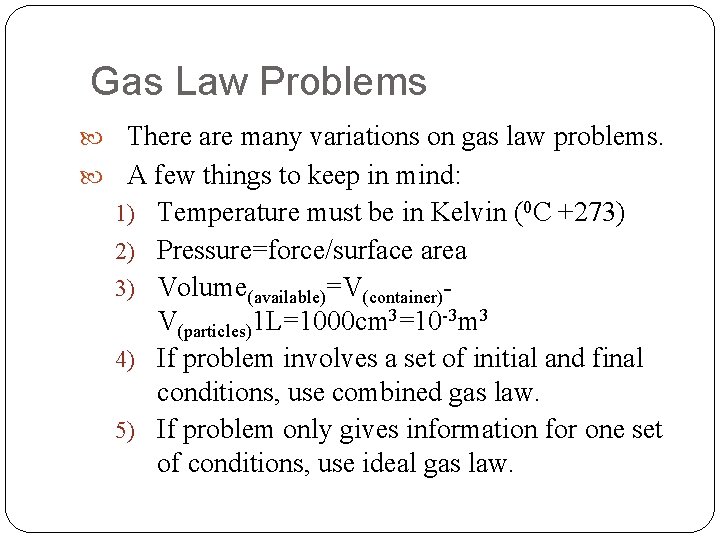
Gas Law Problems There are many variations on gas law problems. A few things to keep in mind: 1) Temperature must be in Kelvin (0 C +273) 2) Pressure=force/surface area 3) Volume(available)=V(container)- V(particles)1 L=1000 cm 3=10 -3 m 3 4) If problem involves a set of initial and final conditions, use combined gas law. 5) If problem only gives information for one set of conditions, use ideal gas law.

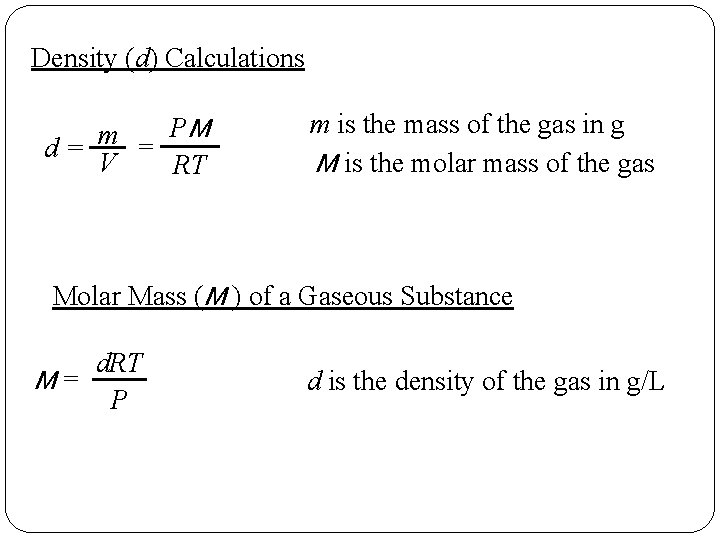
Density (d) Calculations PM m d= = V RT m is the mass of the gas in g M is the molar mass of the gas Molar Mass (M ) of a Gaseous Substance d. RT M= P d is the density of the gas in g/L

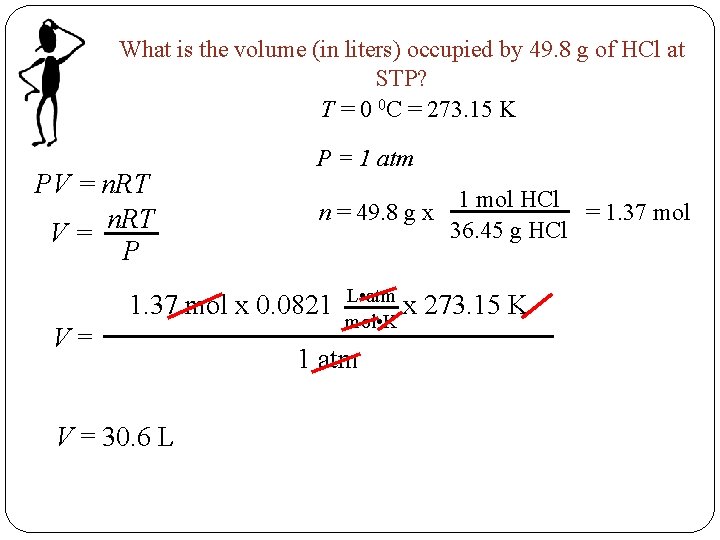
What is the volume (in liters) occupied by 49. 8 g of HCl at STP? T = 0 0 C = 273. 15 K PV = n. RT V= P P = 1 atm n = 49. 8 g x 1. 37 mol x 0. 0821 V= V = 30. 6 L L • atm x mol • K 1 atm 1 mol HCl = 1. 37 mol 36. 45 g HCl 273. 15 K

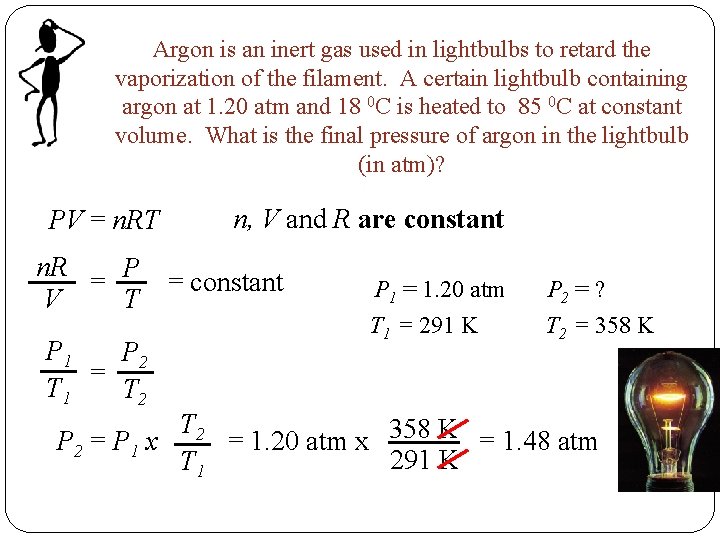
Argon is an inert gas used in lightbulbs to retard the vaporization of the filament. A certain lightbulb containing argon at 1. 20 atm and 18 0 C is heated to 85 0 C at constant volume. What is the final pressure of argon in the lightbulb (in atm)? PV = n. RT n, V and R are constant n. R = P = constant T V P 1 P 2 = T 1 T 2 P 1 = 1. 20 atm T 1 = 291 K P 2 = ? T 2 = 358 K T 2 = 1. 20 atm x 358 K = 1. 48 atm P 2 = P 1 x 291 K T 1

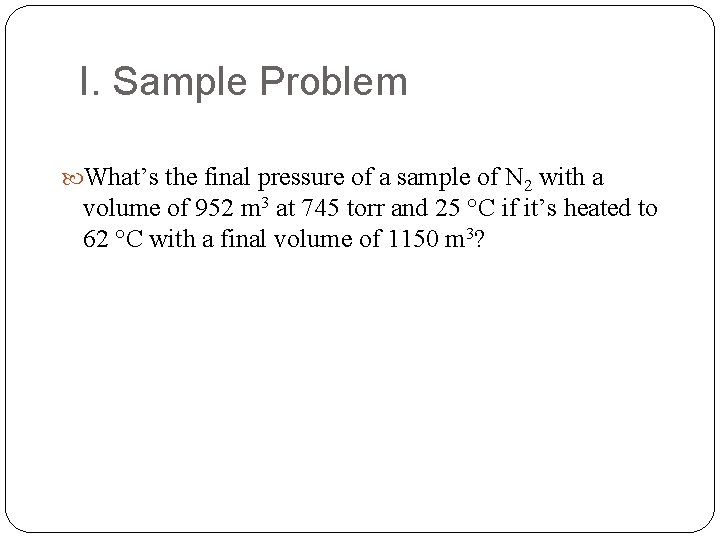
I. Sample Problem What’s the final pressure of a sample of N 2 with a volume of 952 m 3 at 745 torr and 25 °C if it’s heated to 62 °C with a final volume of 1150 m 3?

II Sample Problem What volume, in m. L, does a 0. 245 g sample of N 2 occupy at 21 °C and 750 torr?

III. Sample Problem A sample of N 2 has a volume of 880 m. L and a pressure of 740 torr. What pressure will change the volume to 870 m. L at the same temperature?

Other Uses of Ideal Gas Law The ideal gas law can be used to find other physical values of a gas that are not as obvious. § gas density, d = mass/volume § gas molar mass, MW = mass/mole § stoichiometry, via moles and a balanced equation

VI. Sample Problem Find the density of CO 2(g) at 0 °C and 380 torr.

V. Sample Problem An unknown noble gas was allowed to flow into a 300. 0 m. L glass bulb until the P = 685 torr. Initially, the glass bulb weighed 32. 50 g, but now it weighs 33. 94 g. If the temperature is 27. 0 °C, what’s the identity of the gas?
 Kinetic theory for ideal gases
Kinetic theory for ideal gases Kenetic particle theory
Kenetic particle theory Kinetic theory of gases
Kinetic theory of gases Kinetic theory of gases postulates
Kinetic theory of gases postulates Kinetic theory of gases
Kinetic theory of gases Kinetic molecular theory
Kinetic molecular theory Postulates of kinetic theory of gases
Postulates of kinetic theory of gases Kinetic theory of gases
Kinetic theory of gases Ideal gas constant in kpa
Ideal gas constant in kpa Pseudo reduced specific volume
Pseudo reduced specific volume Imaginary gas
Imaginary gas Differences between ideal gas and real gas
Differences between ideal gas and real gas Ideal gas vs perfect gas
Ideal gas vs perfect gas Difference between ideal gas and real gas
Difference between ideal gas and real gas Characteristics of ideal gases
Characteristics of ideal gases Ideal gas characteristics
Ideal gas characteristics Are ideal gases compressible
Are ideal gases compressible First law of thermodynamics for ideal gas
First law of thermodynamics for ideal gas Facts about montesquieu
Facts about montesquieu Relation between pressure and kinetic energy of gas
Relation between pressure and kinetic energy of gas Kinetic energy of gas formula
Kinetic energy of gas formula Grahams law
Grahams law Molecular theory of gases and liquids
Molecular theory of gases and liquids Immiscible
Immiscible Gas laws crash course
Gas laws crash course Direct vs indirect relationship
Direct vs indirect relationship All the gas laws
All the gas laws What is the combined gas law
What is the combined gas law All the gas laws
All the gas laws Different gas laws
Different gas laws Combined gas law
Combined gas law Gas law conceptual questions
Gas law conceptual questions Mathematical formula for charles law
Mathematical formula for charles law How to solve ideal gas law
How to solve ideal gas law 3 gas laws
3 gas laws Different gas laws
Different gas laws