RAOULTS LAW The partial vapour pressure of a















- Slides: 38


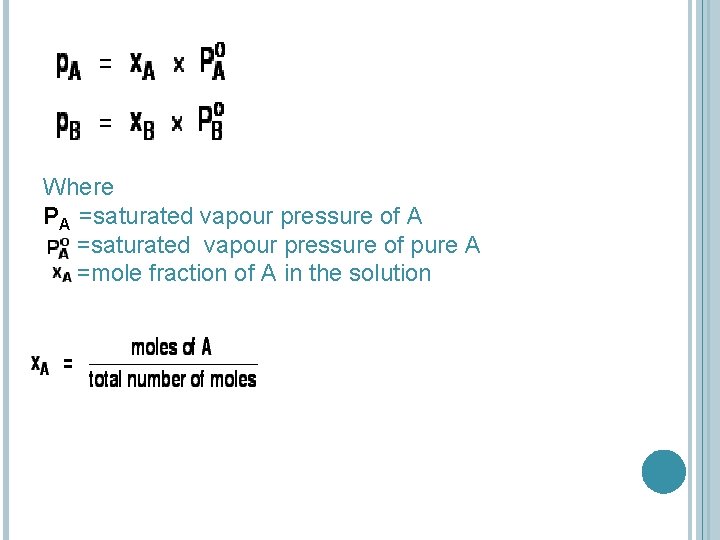
RAOULT’S LAW The partial vapour pressure of a component in a mixture is equal to the vapour pressure of the pure component at that temperature multiplied by its mole fraction in the mixture.

Where PA =saturated vapour pressure of pure A =mole fraction of A in the solution

ü Raoult’s Law is obeyed by mixtures of similar compounds they are said to form IDEAL SOLUTIONS. The substances A and B form an ideal solution if the intermolecular forces A----A, A----B and B----B are all equal. ü 1. 2. Examples of ideal mixtures are hexane and heptane benzene and methylbenzene 3. propan-1 -ol and propan-2 -ol

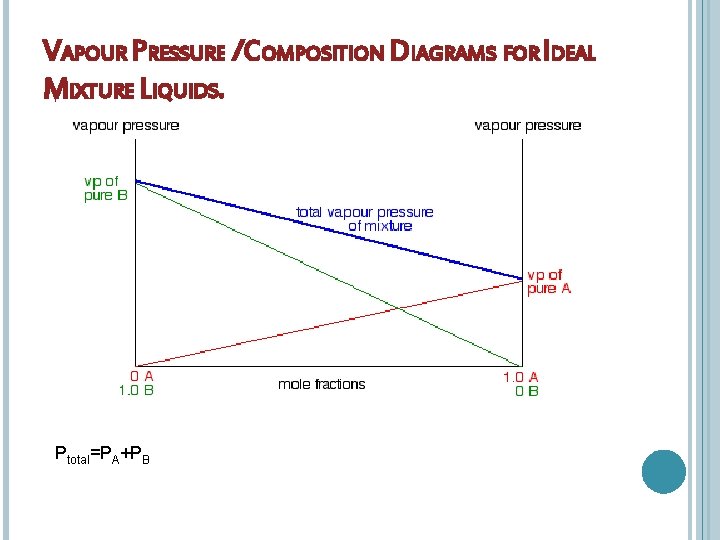
VAPOUR PRESSURE /COMPOSITION DIAGRAMS FOR IDEAL MIXTURE LIQUIDS. Ptotal=PA+PB

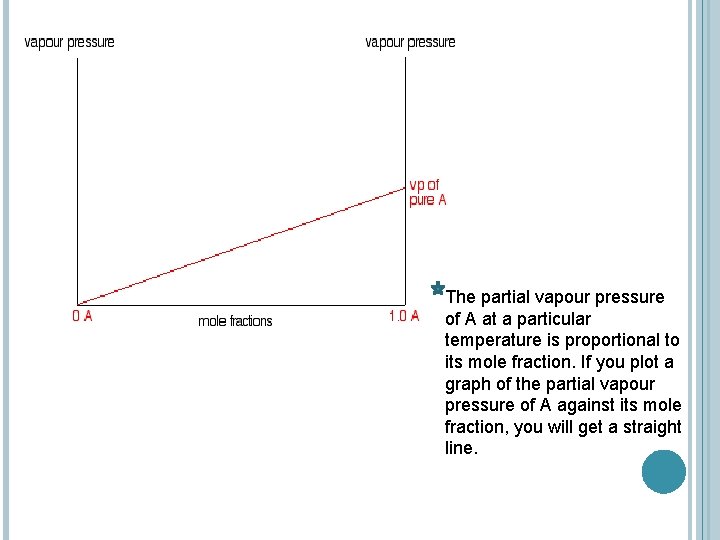
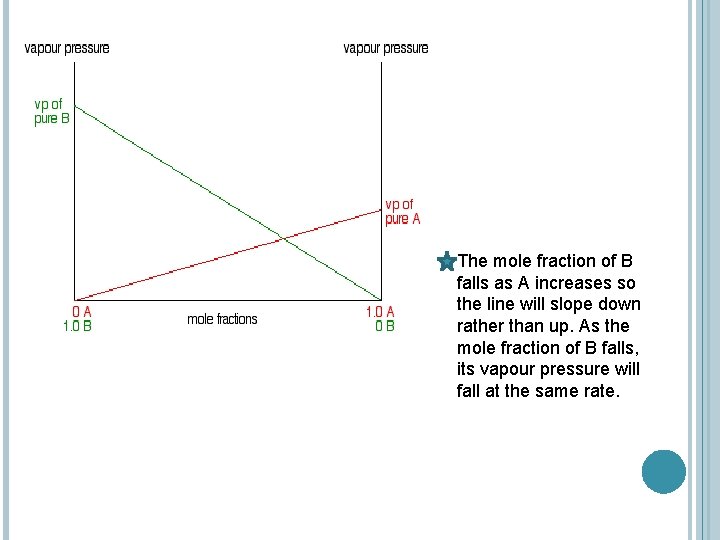
The partial vapour pressure of A at a particular temperature is proportional to its mole fraction. If you plot a graph of the partial vapour pressure of A against its mole fraction, you will get a straight line.

The mole fraction of B falls as A increases so the line will slope down rather than up. As the mole fraction of B falls, its vapour pressure will fall at the same rate.

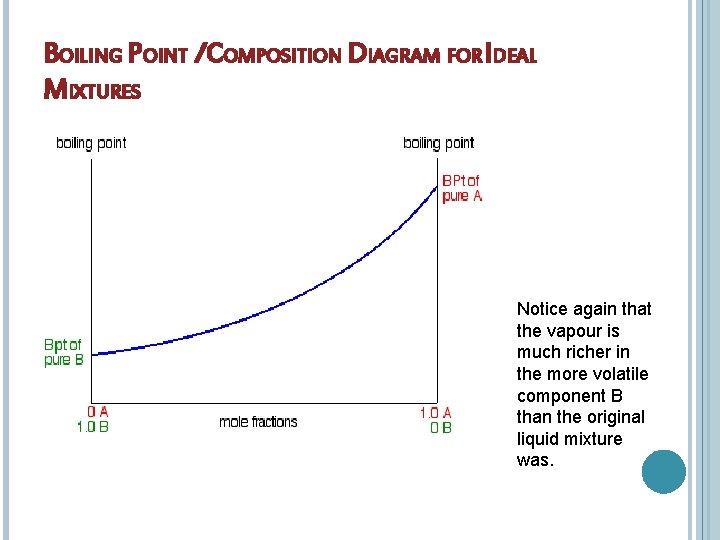
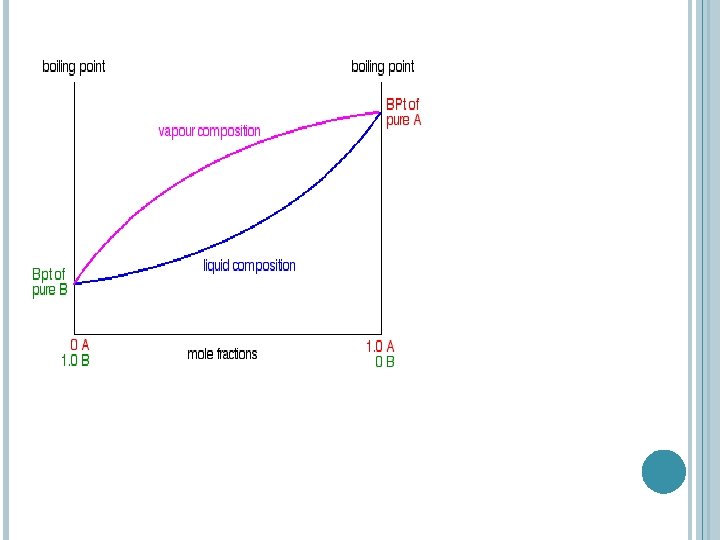
BOILING POINT /COMPOSITION DIAGRAM FOR IDEAL MIXTURES Notice again that the vapour is much richer in the more volatile component B than the original liquid mixture was.


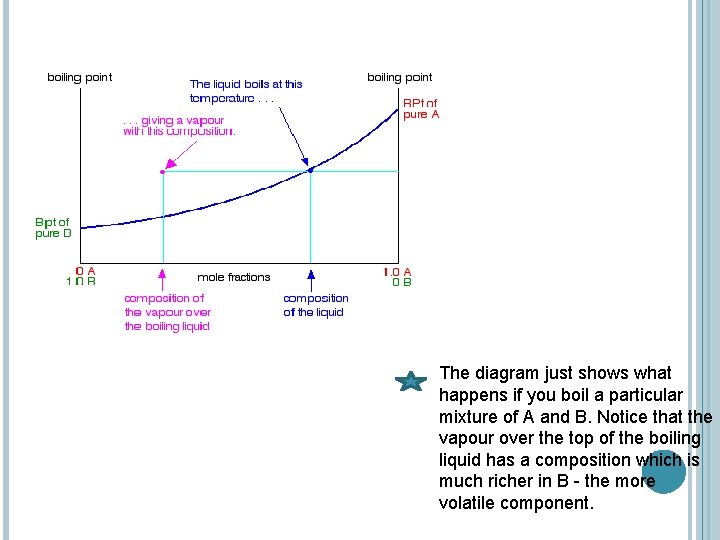
The diagram just shows what happens if you boil a particular mixture of A and B. Notice that the vapour over the top of the boiling liquid has a composition which is much richer in B - the more volatile component.

Solutions of liquids which do not obey Raoult's Law are called non-ideal solutions. There are two types of non ideal solutions 1. positive Deviation from Raoult's law 2. Negative Deviation From Raoult’s Law

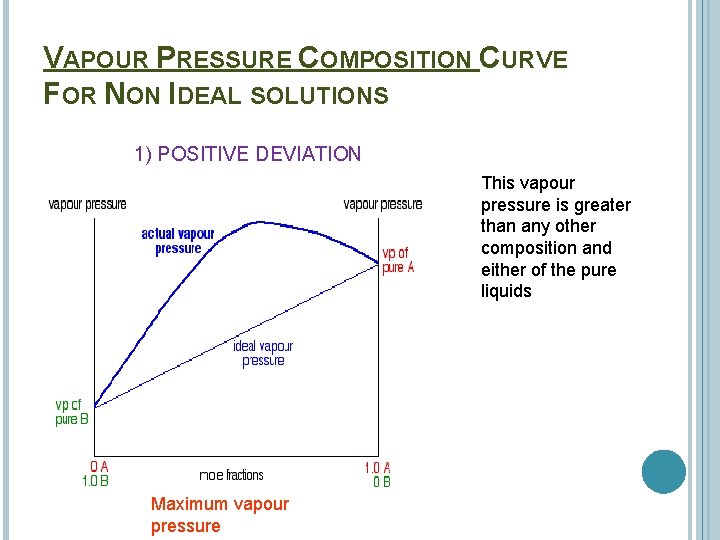
Solutions which have a vapour pressure greater than that predicted from Raoult’s Law are said to show a positive deviation from the law. E. g. Hexane and ethanol This is where the A--B interaction is weaker than the A--A and the B--B interactions. As a result the molecules escape from the mixture more easily than for an ideal solution.

VAPOUR PRESSURE COMPOSITION CURVE FOR NON IDEAL SOLUTIONS 1) POSITIVE DEVIATION This vapour pressure is greater than any other composition and either of the pure liquids Maximum vapour pressure

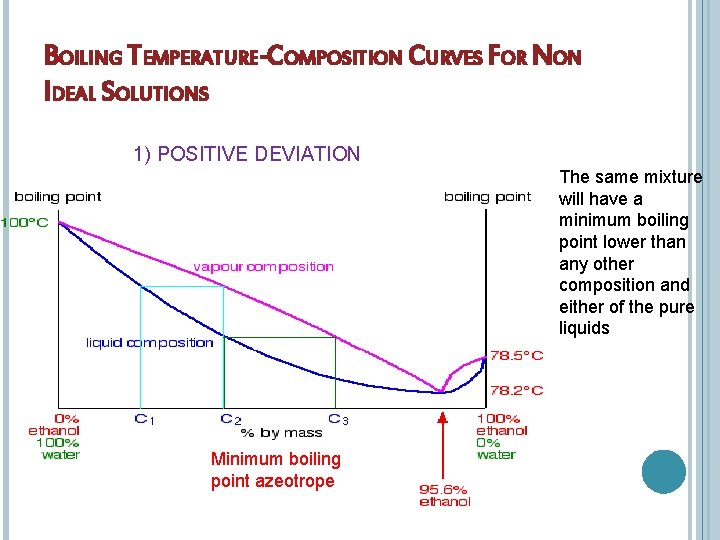
BOILING TEMPERATURE-COMPOSITION CURVES FOR NON IDEAL SOLUTIONS 1) POSITIVE DEVIATION The same mixture will have a minimum boiling point lower than any other composition and either of the pure liquids Minimum boiling point azeotrope

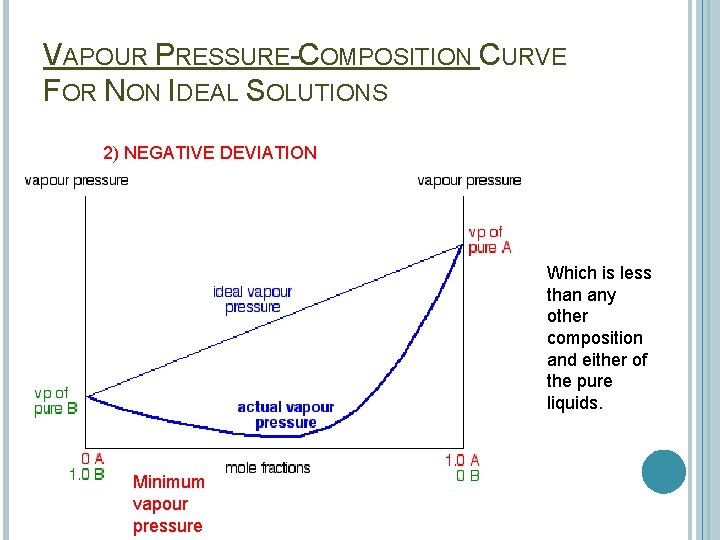
Solution with a vapour pressure lower than the calculated values are said to show a negative deviation. E. g. Nitric acid and water This is where the A--B interaction is greater than the A--A and the B--B interactions. It is more difficult for the molecules to escape from the mixture than for an ideal mixture.

VAPOUR PRESSURE-COMPOSITION CURVE FOR NON IDEAL SOLUTIONS 2) NEGATIVE DEVIATION Which is less than any other composition and either of the pure liquids. Minimum vapour pressure

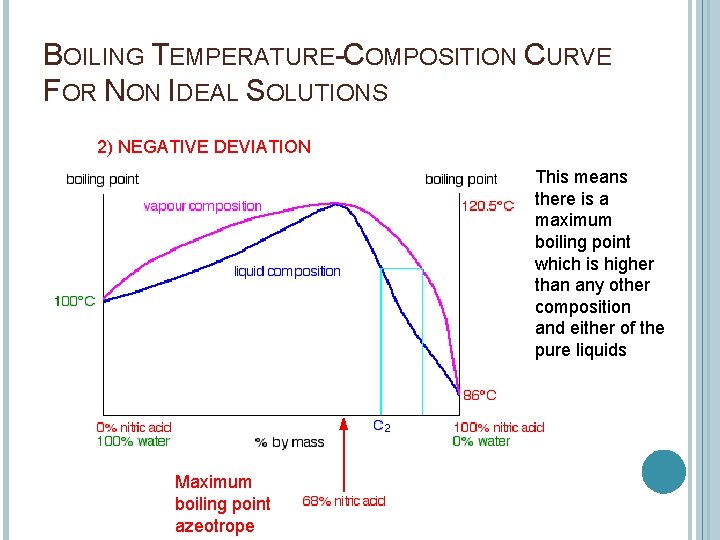
BOILING TEMPERATURE-COMPOSITION CURVE FOR NON IDEAL SOLUTIONS 2) NEGATIVE DEVIATION This means there is a maximum boiling point which is higher than any other composition and either of the pure liquids Maximum boiling point azeotrope

NOTE THE TERMS USED Minimum boiling point azeotrope Maximum boiling point azeotrope

SIMPLE DISTILLATION Simple distillation is designed to evaporate a volatile liquid from a solution of non-volatile substances; the vapour is then condensed in the water condenser and collected in the receiver.

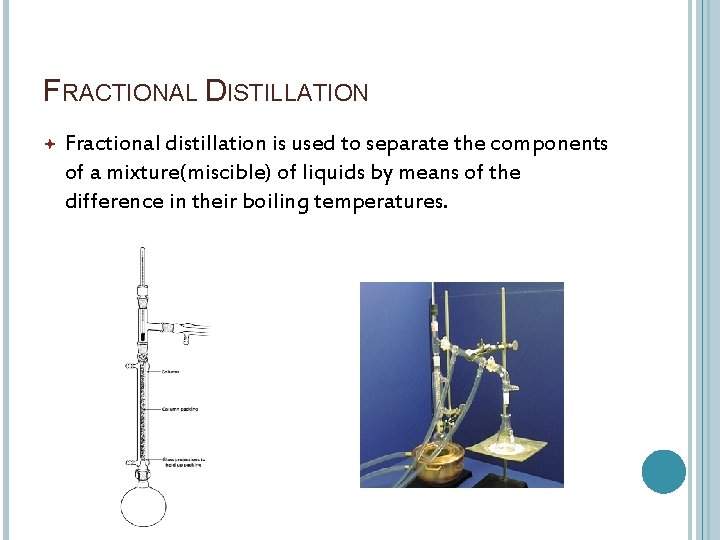
FRACTIONAL DISTILLATION Fractional distillation is used to separate the components of a mixture(miscible) of liquids by means of the difference in their boiling temperatures.

A mixture rich in the most volatile component distils over at the top of the column, where thermometer registers its boiling temperature. As distillation continues the temperature rises towards the boiling temperature of the next most volatile component. The receiver is changed to collect the second component.

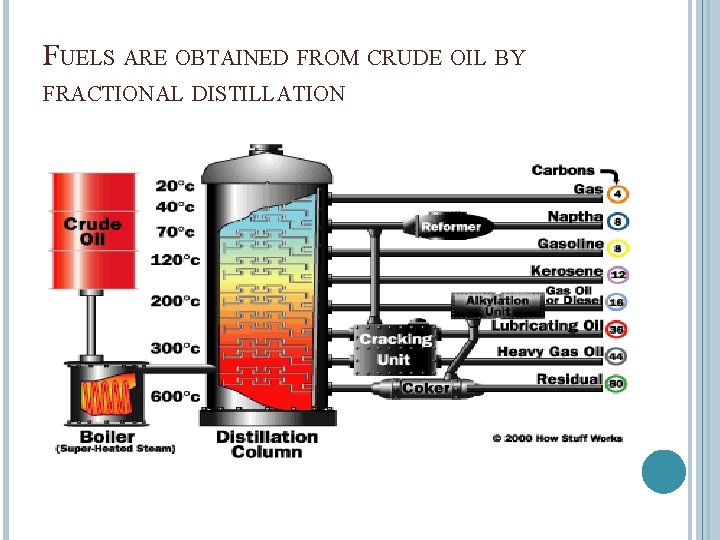
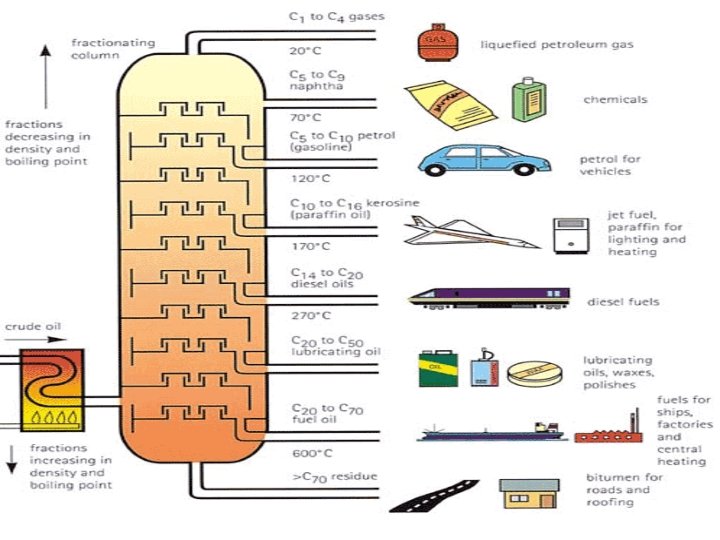
FUELS ARE OBTAINED FROM CRUDE OIL BY FRACTIONAL DISTILLATION


DISTILLATION AT REDUCED PRESSURE High boiling liquids and many liquids which have a tendency to decompose near their boiling temperatures are often purified by distillation under reduced pressure, since lowering the pressure dramatically reduces the temperature at which a liquid will distil. Distillation under reduced pressure always carries a slight risk of the apparatus *imploding -to collapse inwardly with force as a result of the external pressure being greater than the internal pressure, or cause something to collapse inwardly

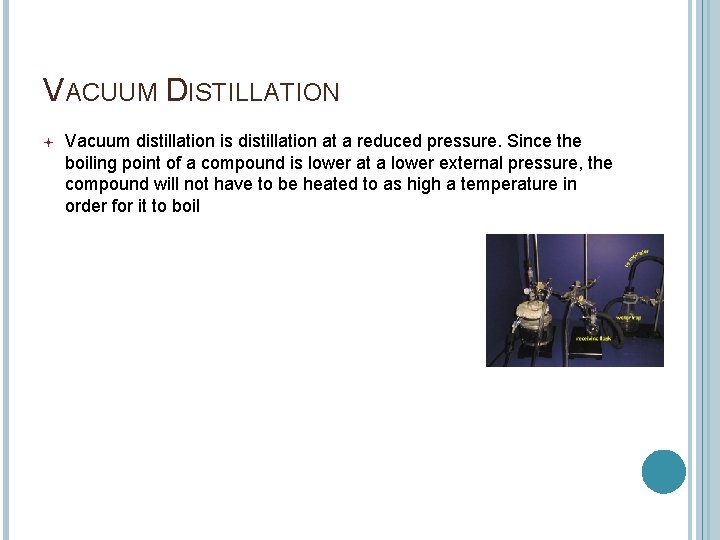
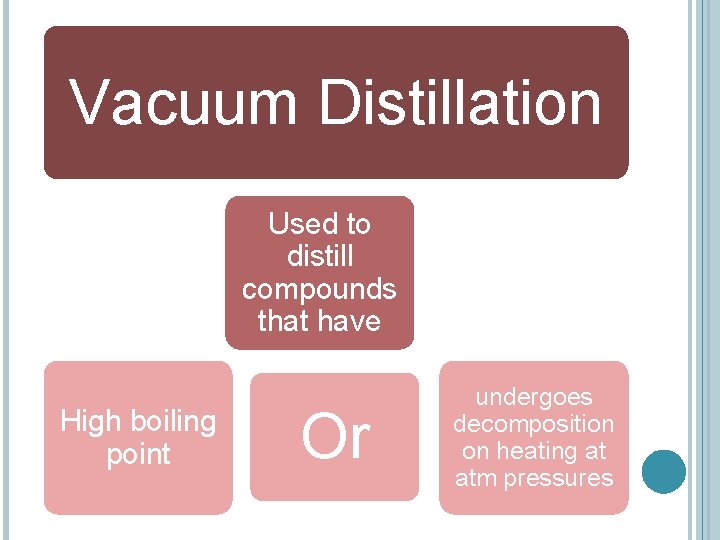
VACUUM DISTILLATION Vacuum distillation is distillation at a reduced pressure. Since the boiling point of a compound is lower at a lower external pressure, the compound will not have to be heated to as high a temperature in order for it to boil

Vacuum Distillation Used to distill compounds that have High boiling point Or undergoes decomposition on heating at atm pressures

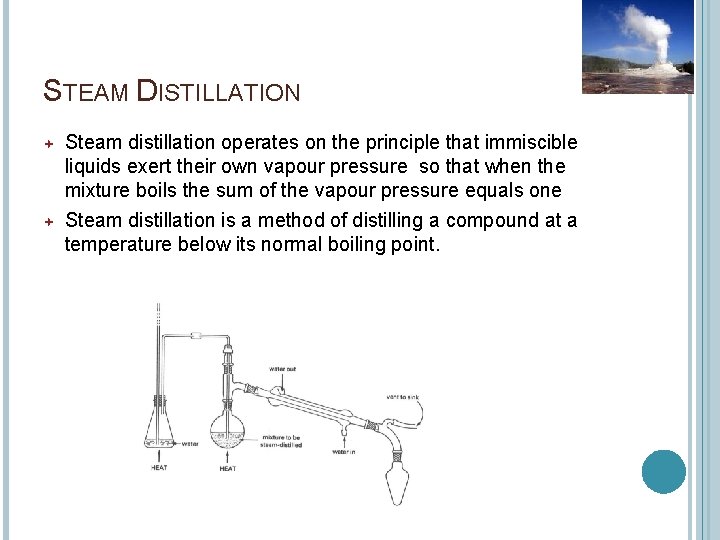
STEAM DISTILLATION Steam distillation operates on the principle that immiscible liquids exert their own vapour pressure so that when the mixture boils the sum of the vapour pressure equals one Steam distillation is a method of distilling a compound at a temperature below its normal boiling point.

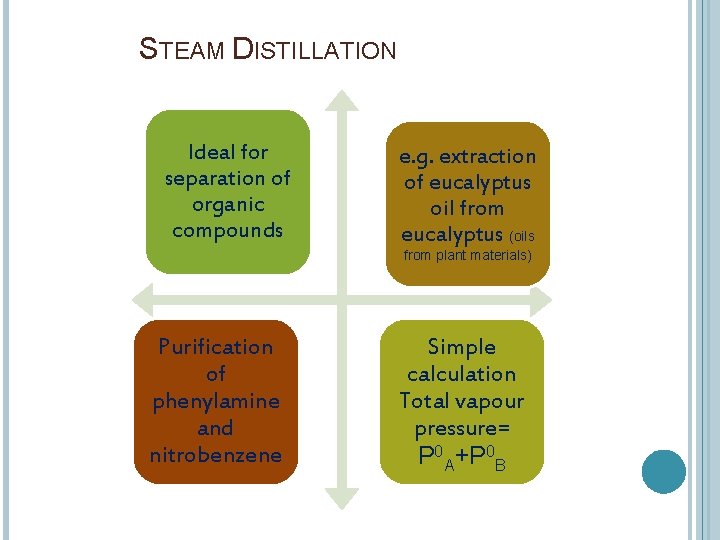
STEAM DISTILLATION Ideal for separation of organic compounds e. g. extraction of eucalyptus oil from eucalyptus (oils from plant materials) Purification of phenylamine and nitrobenzene Simple calculation Total vapour pressure= P 0 A+P 0 B

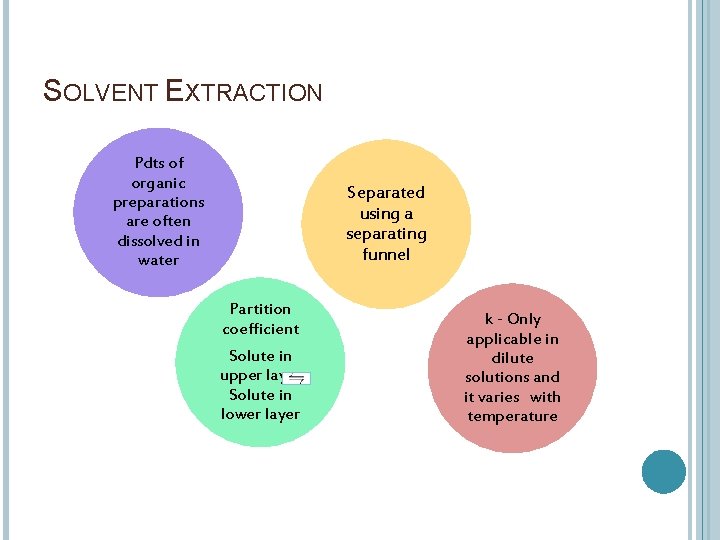
SOLVENT EXTRACTION Liquids that form two layers when mixed provide an opportunity for purification of materials that prefer one layer more than the other. For example, many organic chemicals are liquids that are very non-polar and separate from water because it is quite polar.

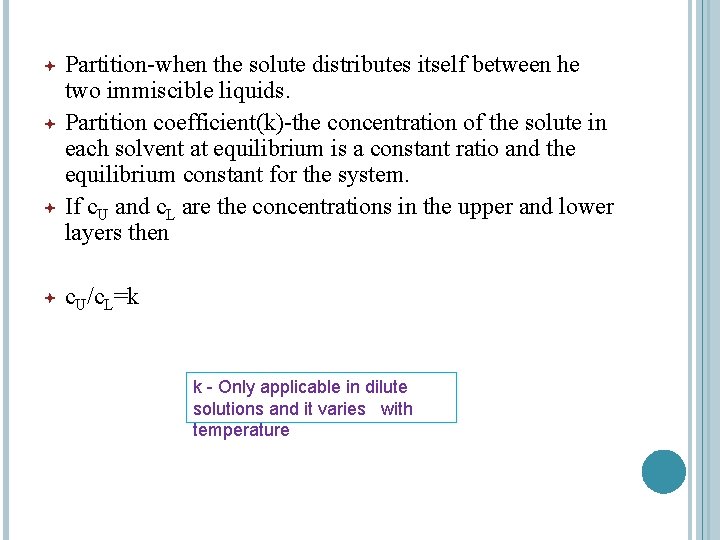
Partition-when the solute distributes itself between he two immiscible liquids. Partition coefficient(k)-the concentration of the solute in each solvent at equilibrium is a constant ratio and the equilibrium constant for the system. If c. U and c. L are the concentrations in the upper and lower layers then c. U/c. L=k k - Only applicable in dilute solutions and it varies with temperature

THE PARTITION COEFFICIENT WILL REMAIN CONSTANT UNDER THESE CONDITIONS: 1. the temperature is constant 2. the solvents are immiscible and do not react with each other 3. The solute does not react associate or dissociate in solvents.

SOLVENT EXTRACTION Pdts of organic preparations are often dissolved in water Separated using a separating funnel Partition coefficient Solute in upper layer Solute in lower layer k - Only applicable in dilute solutions and it varies with temperature

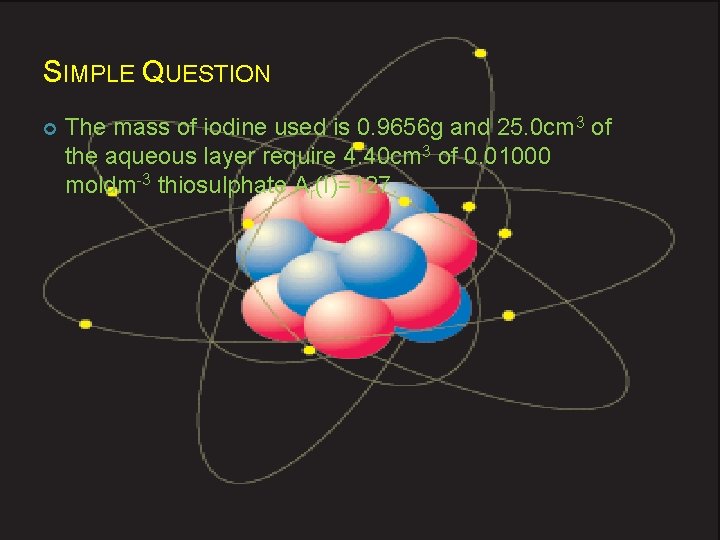
SIMPLE QUESTION The mass of iodine used is 0. 9656 g and 25. 0 cm 3 of the aqueous layer require 4. 40 cm 3 of 0. 01000 moldm-3 thiosulphate Ar(I)=127.

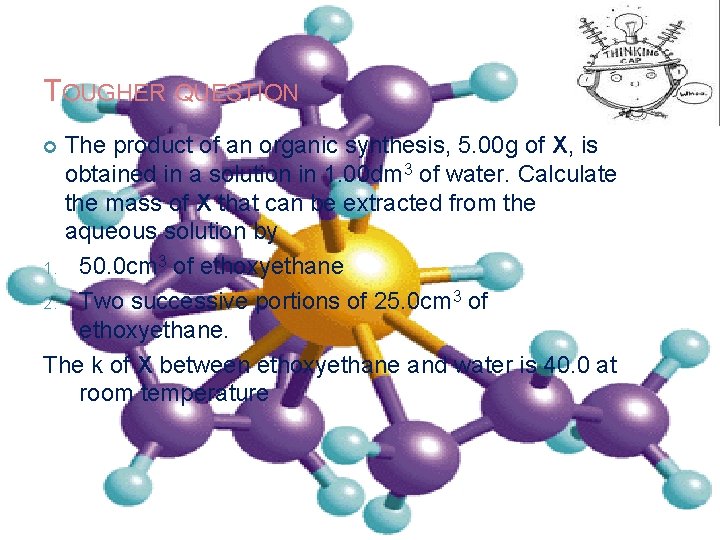
TOUGHER QUESTION The product of an organic synthesis, 5. 00 g of X, is obtained in a solution in 1. 00 dm 3 of water. Calculate the mass of X that can be extracted from the aqueous solution by 1. 50. 0 cm 3 of ethoxyethane 2. Two successive portions of 25. 0 cm 3 of ethoxyethane. The k of X between ethoxyethane and water is 40. 0 at room temperature

INDUSTRIAL APPLICATIONS OF DISTILLATION Petroleum Rum Fragrance

PETROLEUM

• RUM • BEER • VODKA

Perfumes and Fragrances