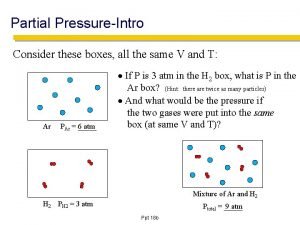
Gases GASES manometers Kinetic theory of gases pressure










- Slides: 18

Gases

GASES manometers Kinetic theory of gases pressure Units of pressure Behavior of gases Pressure vs. volume Pressure vs. temperature Combined gas law Ideal gas law Temperature vs. volume Partial pressure of a gas Diffusion/effusion

Diffusion and Effusion • Diffusion – The gradual mixing of 2 gases due to random spontaneous motion • Effusion – When molecules of a confined gas escape through a tiny opening in a container

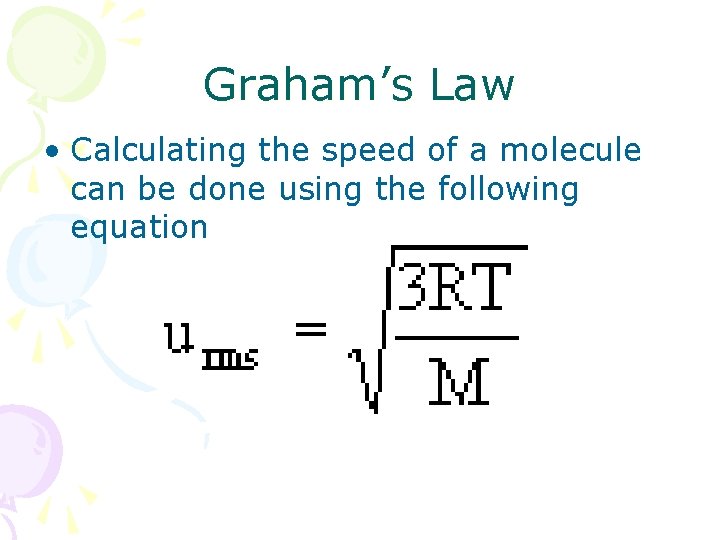
Graham’s Law

Graham’s Law • Conceptual: • Lighter gases travel quicker at the same temp • Consider H 2 vs. Cl 2 Which would diffuse at the greater velocity?


Graham’s Law • Calculating the speed of a molecule can be done using the following equation

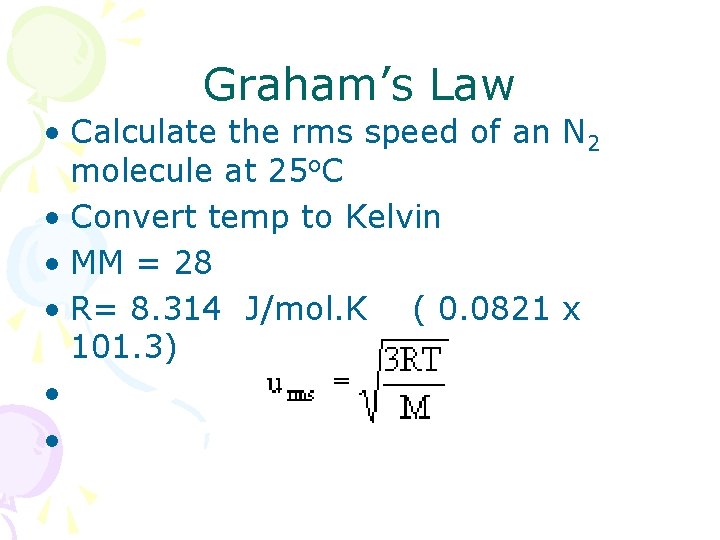
Graham’s Law • Calculate the rms speed of an N 2 molecule at 25 o. C • Convert temp to Kelvin • MM = 28 • R= 8. 314 J/mol. K ( 0. 0821 x 101. 3) • •

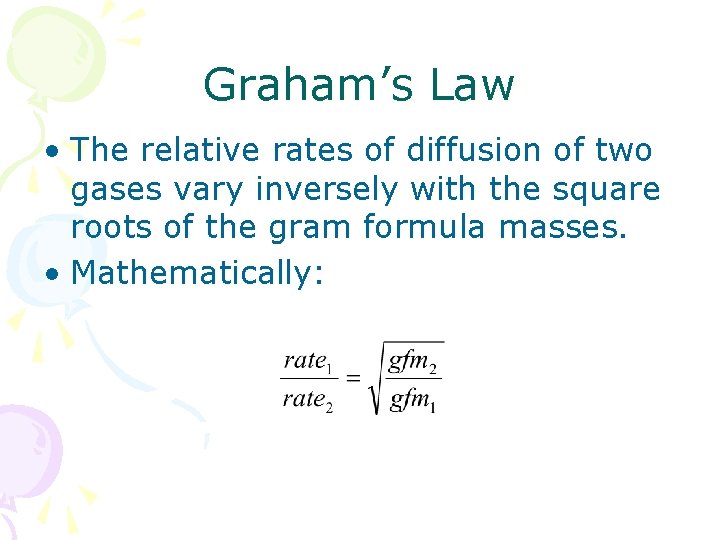
Graham’s Law • The relative rates of diffusion of two gases vary inversely with the square roots of the gram formula masses. • Mathematically:

Graham’s Law Problem • A helium atom travels an average 1000. m/s at 250 o. C. How fast would an atom of radon travel at the same temperature? • Solution: – Let rate 1 = x rate 2 = 1000. m/s – Gfm 1 = radon 222 g/mol – Gfm 2 = helium = 4. 00 g/mol

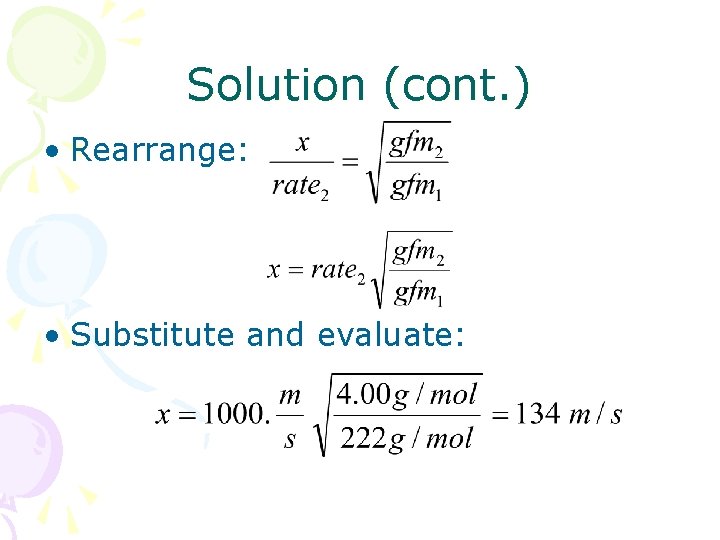
Solution (cont. ) • Rearrange: • Substitute and evaluate:

Applications of Graham’s Law • Separation of uranium isotopes – 235 U – Simple, inexpensive technique – Used in Iraq in early 1990’s as part of nuclear weapons development program • Identifying unknowns – Use relative rates to find gfm

Problem 2 • An unknown gas effuses through an opening at a rate 3. 16 times slower than that of helium gas. What is the gfm of this unknown gas?

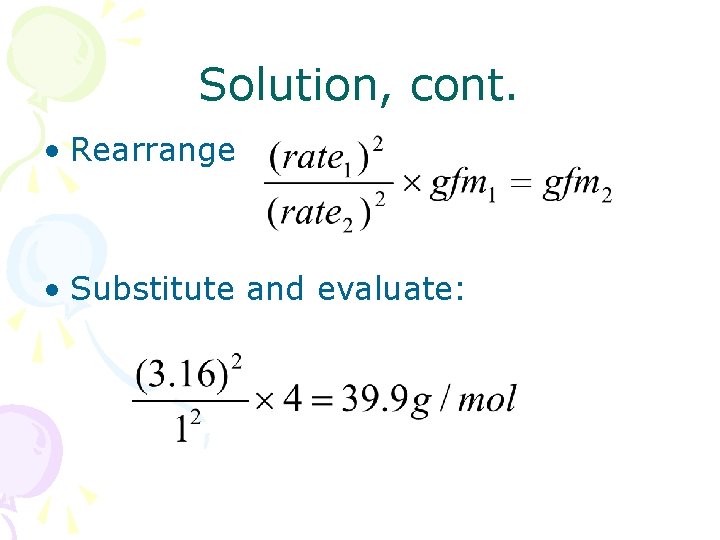
Solution • Let gfm 2 = x gfm 1 = 4. 00 rate 2 = 1 rate 1 = 3. 16 • From Graham’s Law,

Solution, cont. • Rearrange • Substitute and evaluate:

WWWW we do • What is the rms speed of an He atom at 40 o. C

• The rate of effusion was measured for an unknown gas is 10 time slower than helium so • Runknown /R He = 0. 1 what is the molecular mass of the unknown gas

 Kinetic molecular theory
Kinetic molecular theory Particle theory evaporation
Particle theory evaporation Kinetic theory of gases
Kinetic theory of gases Three postulates of kinetic theory of gases
Three postulates of kinetic theory of gases Kinetic theory of gases
Kinetic theory of gases Write postulates of kinetic theory of gases
Write postulates of kinetic theory of gases Postulates of kinetic theory of gases
Postulates of kinetic theory of gases Kinetic theory
Kinetic theory Relation between pressure and kinetic energy of gas
Relation between pressure and kinetic energy of gas Gas pressure
Gas pressure Do gases exert pressure on whatever surrounds them
Do gases exert pressure on whatever surrounds them Wiberg patella
Wiberg patella Pressure support vs pressure control
Pressure support vs pressure control Continuous bedside pressure mapping
Continuous bedside pressure mapping Intrapulmonary pressure
Intrapulmonary pressure Oncotic vs osmotic
Oncotic vs osmotic Partial pressure formula
Partial pressure formula Intrapleural pressure
Intrapleural pressure How are metamorphic rocks classified
How are metamorphic rocks classified