Gases GASES manometers Kinetic theory of gases pressure

















- Slides: 34

Gases

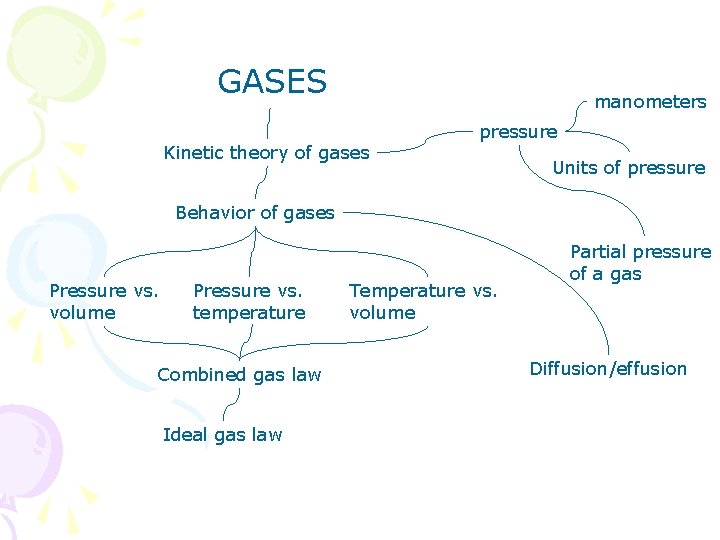
GASES manometers Kinetic theory of gases pressure Units of pressure Behavior of gases Pressure vs. volume Pressure vs. temperature Combined gas law Ideal gas law Temperature vs. volume Partial pressure of a gas Diffusion/effusion

Properties of Gases • Very low density • Low freezing points • Low boiling points • Can diffuse (rapidly and spontaneously spread out and mix) • Flow • Expand to fill container • Compressible

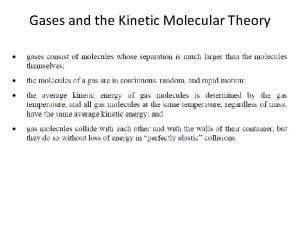
Kinetic Molecular Theory of Gases • Particles move non-stop, in straight lines. • Particles have negligible volume (treat as points) • Particles have no attractions to each other (no repulsions, either). • Collisions between particles are “elastic” (no gain or loss of energy) • Particles exert pressure on the container by colliding with the container walls.

Kinetic Energy • Energy due to motion • KE = ½ mv 2

Temperature • Temperature is a measure of average kinetic energy. – Temperature measures how quickly the particles are moving. (Heat IS NOT the same as temperature!) – If temperature increases, kinetic energy increases. • Which has greater kinetic energy: a 25 g sample of water at 25 o. C or a 25 g sample of water at -15 o. C?

Why use the Kelvin scale? • In the Kelvin scale, there is an absolute correlation between temperature and kinetic energy. – As temperature in Kelvin increases, kinetic energy increases. • Absolute zero: All molecular motion ceases. There is no kinetic energy. – 0 K

Kelvin-Celsius Conversions • K = o. C + 273. 15 • o. C = K – 273. 15

Kelvin-Celsius conversions • The temperature of liquid nitrogen is -196 o. C. What is this temperature in Kelvin? • Convert 872 Kelvin to Celsius temperature.

Pressure • Pressure = force/area • Atmospheric pressure – Because air molecules collide with objects • More collisions greater pressure

Pressure Units • Atmosphere • Pounds per square inch (psi) • mm Hg • Torr • Pascal (Pa) or kilopascal (k. Pa) 1 atm = 14. 7 psi = 760 mm Hg = 760 torr = 101. 3 k. Pa

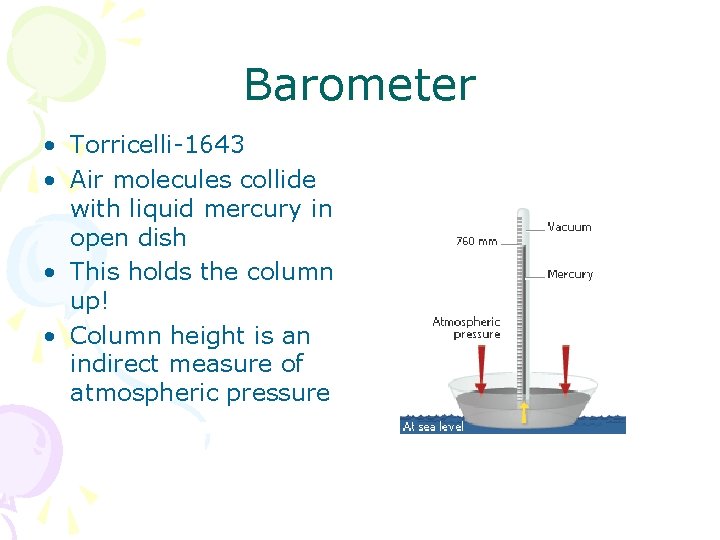
Barometer • Torricelli-1643 • Air molecules collide with liquid mercury in open dish • This holds the column up! • Column height is an indirect measure of atmospheric pressure

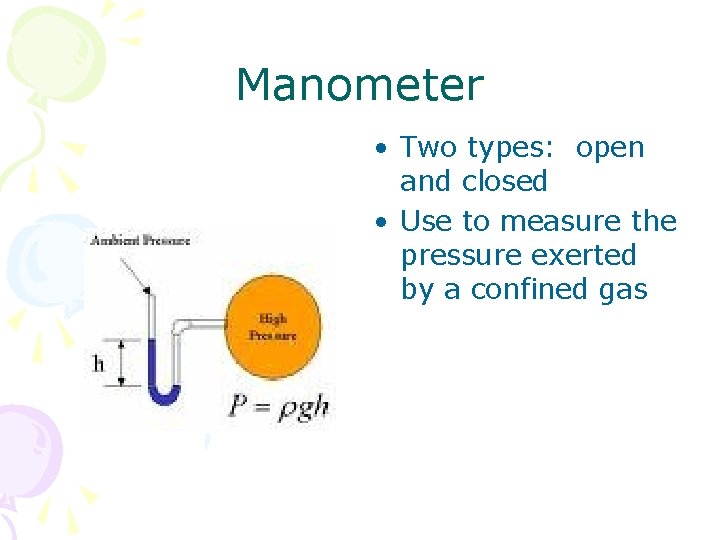
Manometer • Two types: open and closed • Use to measure the pressure exerted by a confined gas

Chapter 15 Wrapup (Honors) • At the same temperature, smaller molecules (i. e. , molecules with lower gfm) have faster average velocity. • Energy flows from warmer objects to cooler objects. • Plasma – High energy state consisting of cations and electrons • Found in sun, fluorescent lights

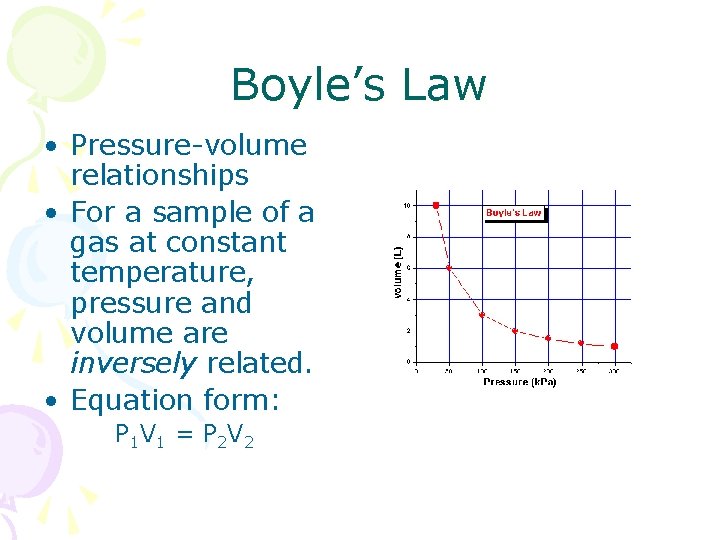
Boyle’s Law • Pressure-volume relationships • For a sample of a gas at constant temperature, pressure and volume are inversely related. • Equation form: P 1 V 1 = P 2 V 2

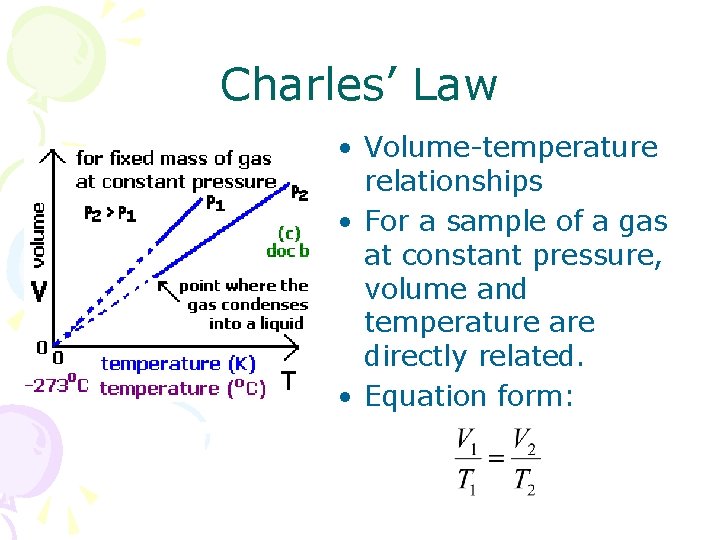
Charles’ Law • Volume-temperature relationships • For a sample of a gas at constant pressure, volume and temperature are directly related. • Equation form:

Guy-Lussac’s Law • Pressure temperature relationships • For a sample of a confined gas at constant volume, temperature and pressure are directly related.

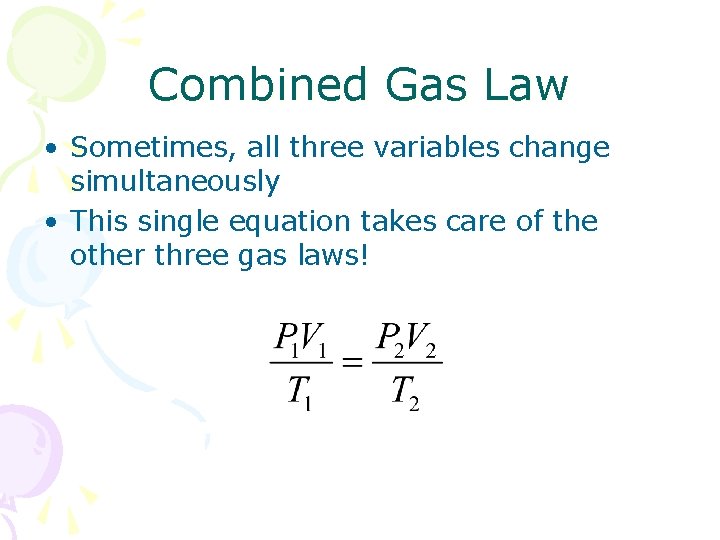
Combined Gas Law • Sometimes, all three variables change simultaneously • This single equation takes care of the other three gas laws!

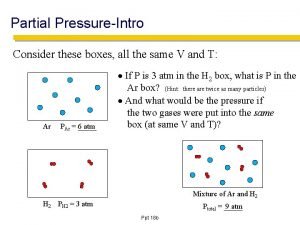
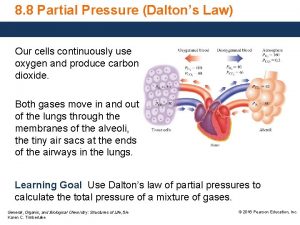
Dalton’s Law of Partial Pressures • For a mixture of (nonreacting) gases, the total pressure exerted by the mixture is equal to the sum of the pressures exerted by the individual gases.

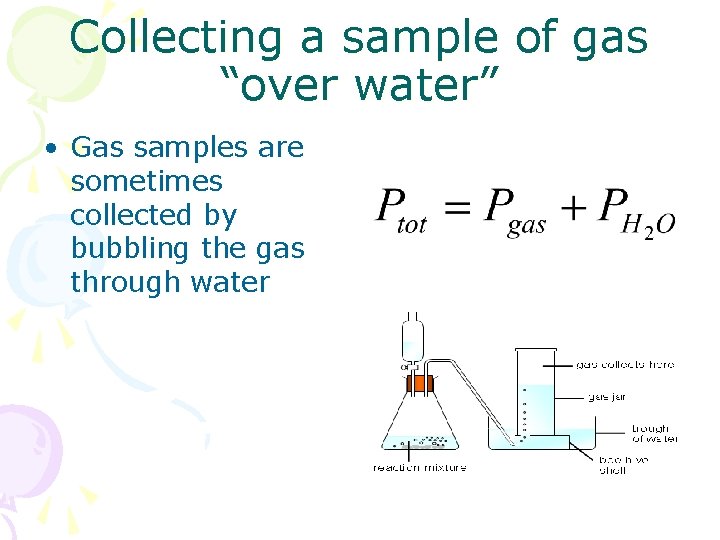
Collecting a sample of gas “over water” • Gas samples are sometimes collected by bubbling the gas through water

• If a question asks about something relating to a “dry gas”, Dalton’s Law must be used to correct for the vapor pressure of water! Table: Vapor Pressure of Water

Ideal Gas Law • The number of moles of gas affects pressure and volume, also! – n, number of moles • • • n V n P P 1/V P T V T Where R is the universal gas constant R = 0. 0821 L●atm/mol●K

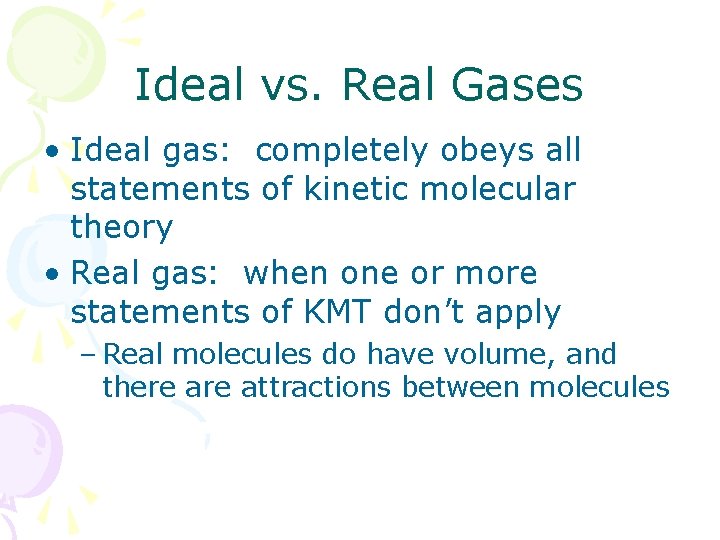
Ideal vs. Real Gases • Ideal gas: completely obeys all statements of kinetic molecular theory • Real gas: when one or more statements of KMT don’t apply – Real molecules do have volume, and there attractions between molecules

When to expect ideal behavior? • Gases are most likely to exhibit ideal behavior at… – High temperatures – Low pressures • Gases are most likely to exhibit real (i. e. , non-ideal) behavior at… – Low temperatures – High pressures

Diffusion and Effusion • Diffusion – The gradual mixing of 2 gases due to random spontaneous motion • Effusion – When molecules of a confined gas escape through a tiny opening in a container

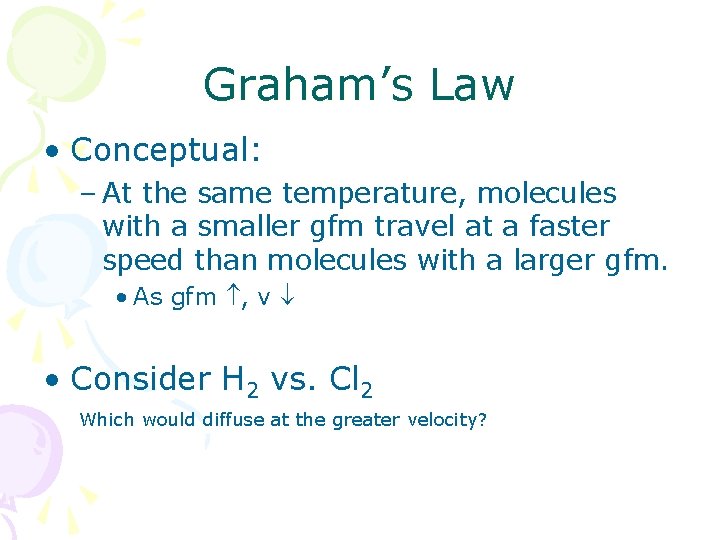
Graham’s Law • Thomas Graham (1805 -1869) • Do all gases diffuse at the same rate? • Graham’s law discusses this quantitatively. • Technically, this law only applies to gases effusing into a vacuum or into each other.

Graham’s Law • Conceptual: – At the same temperature, molecules with a smaller gfm travel at a faster speed than molecules with a larger gfm. • As gfm , v • Consider H 2 vs. Cl 2 Which would diffuse at the greater velocity?

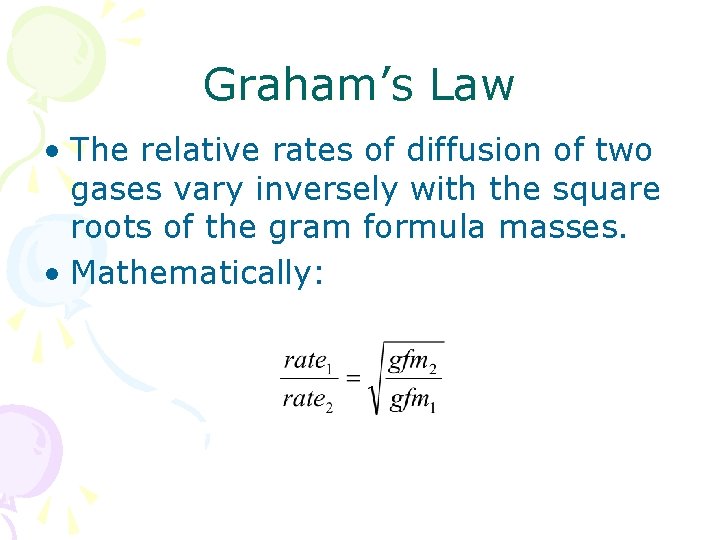
Graham’s Law • The relative rates of diffusion of two gases vary inversely with the square roots of the gram formula masses. • Mathematically:

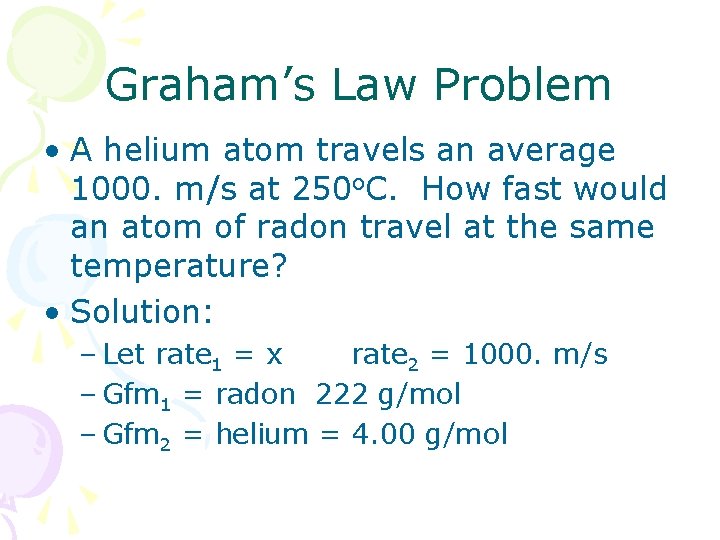
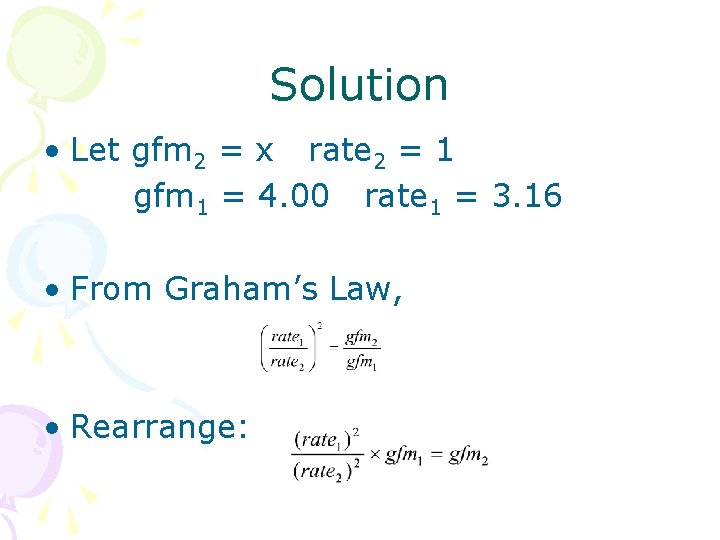
Graham’s Law Problem • A helium atom travels an average 1000. m/s at 250 o. C. How fast would an atom of radon travel at the same temperature? • Solution: – Let rate 1 = x rate 2 = 1000. m/s – Gfm 1 = radon 222 g/mol – Gfm 2 = helium = 4. 00 g/mol

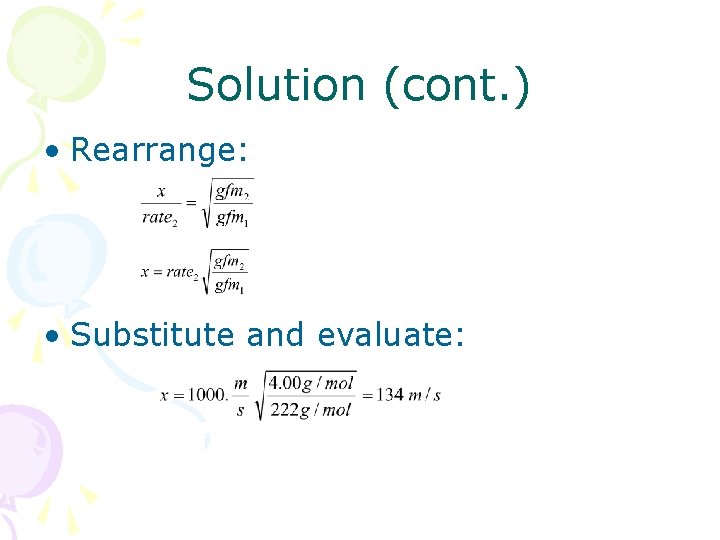
Solution (cont. ) • Rearrange: • Substitute and evaluate:

Applications of Graham’s Law • Separation of uranium isotopes – 235 U – Simple, inexpensive technique – Used in Iraq in early 1990’s as part of nuclear weapons development program • Identifying unknowns – Use relative rates to find gfm

Problem 2 • An unknown gas effuses through an opening at a rate 3. 16 times slower than that of helium gas. What is the gfm of this unknown gas?

Solution • Let gfm 2 = x rate 2 = 1 gfm 1 = 4. 00 rate 1 = 3. 16 • From Graham’s Law, • Rearrange:

Solution, cont. • Substitute and evaluate:
 Kinetic molecular theory of gases
Kinetic molecular theory of gases Kenetic particle theory
Kenetic particle theory Kinetic theory of gases
Kinetic theory of gases Kinetic theory of gases postulates
Kinetic theory of gases postulates Kinetic theory of gases
Kinetic theory of gases Write postulates of kinetic theory of gases
Write postulates of kinetic theory of gases Write the postulates of kinetic theory of gases
Write the postulates of kinetic theory of gases General gas equation is
General gas equation is Relation between pressure and kinetic energy of gas
Relation between pressure and kinetic energy of gas Do gases exert pressure on whatever surrounds them
Do gases exert pressure on whatever surrounds them Do gases exert pressure on whatever surrounds them
Do gases exert pressure on whatever surrounds them Patella baja
Patella baja Pressure support vs pressure control
Pressure support vs pressure control Continuous bedside pressure mapping
Continuous bedside pressure mapping Intrapleural pressure
Intrapleural pressure Oncotic vs osmotic
Oncotic vs osmotic Hypergraph containers
Hypergraph containers Clubbing fingers
Clubbing fingers Metamorphic grade
Metamorphic grade Sore throat after surgery
Sore throat after surgery Stream line equation
Stream line equation Factors affecting gfr
Factors affecting gfr Oncotic pressure vs hydrostatic pressure
Oncotic pressure vs hydrostatic pressure Low oncotic pressure
Low oncotic pressure Oncotic pressure vs hydrostatic pressure
Oncotic pressure vs hydrostatic pressure Dynamothermal
Dynamothermal How is blood pressure regulated
How is blood pressure regulated How to find partial pressure from total pressure
How to find partial pressure from total pressure Prvc ventilation
Prvc ventilation High pressure and low pressure
High pressure and low pressure Tiefdruckgebiet
Tiefdruckgebiet Kinetic molecular theory of solids
Kinetic molecular theory of solids The kinetic theory of matter states that
The kinetic theory of matter states that Kinetic theory of matter definition
Kinetic theory of matter definition Kinetic theory of matter
Kinetic theory of matter