Importance of Osmosis and Osmotic Pressure Lecture 2


















- Slides: 34

Importance of Osmosis and Osmotic Pressure Lecture 2 21 -6 -2012

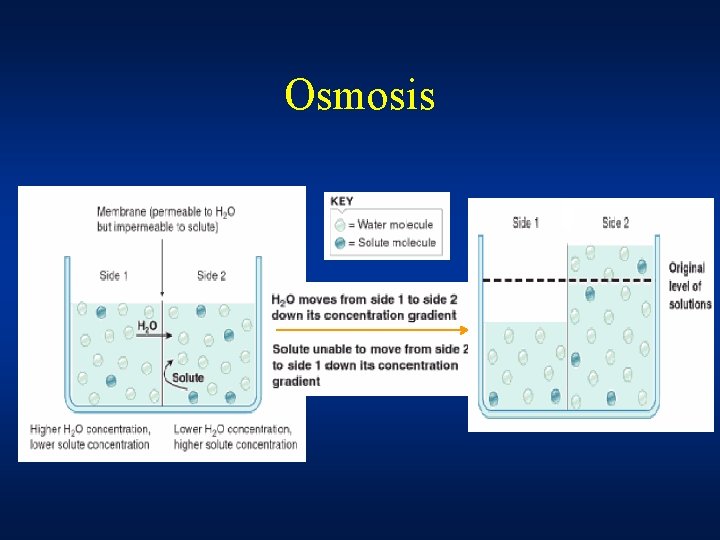
OSMOSIS • Diffusion of water through the semi permeable membrane from a solution of lower concentration towards a solution of higher concentration OR • Water tends to flow from where its chemical potential is higher to where it is lower. Two solutions with different chemical potentials separated by a semipermeable membrane OR • Movement of water from region where net hydrostatic pressure is higher to a region where it is low

Osmosis

Factors on which osmotic pressure depends: Van’t Hoffs Equation • All non penetrable solutes in a solution exerts osmotic pressure • According to Van’t Hoff, osmotic pressure (π) depends on the molar concentration (C) of the solution and the temperature T π = R C T where R is the gas constant

• Osmotic pressure is higher when concentration difference is higher or temperature is higher and the molecular weight is lower • Osmotic pressure depends mainly on the molar concentration or molarity of a solution • Osmotic pressure is a colligative property, meaning that the property depends on the concentration of the solute but not on its identity.

Osmolarity/Osmolality • To describe the total number of osmotically active particles per litre of solution term osmolarity is used • Two solutions can have the same molarity but different osmolarities. • The higher the osmolarity, the greater the osmotic pressure of the solution. • The greater the no of ion/molecule when dissolved greater the osmotic pressure

Pressures of a solution • Osmotic pressure (the pulling pressure) of a solution is the measure of tendency of a solution to pull water into it by osmosis because of the relative concentration of non penetrating solute and water • Hydrostatic pressure of a solution (the pushing pressure) is the pressure exerted by a stationary fluidic part of the solution on an object (semi permeable membrane in case of osmosis)

What happens when two solutions with different hydrostatic and osmotic pressures are combined via a semi permeable membrane? • Water is pulled into the solution with relatively higher pulling tendency (concentrated solution). Osmosis takes place • Water is pushed out from the solution with relatively higher pushing tendency (with high water content). Osmosis is opposed

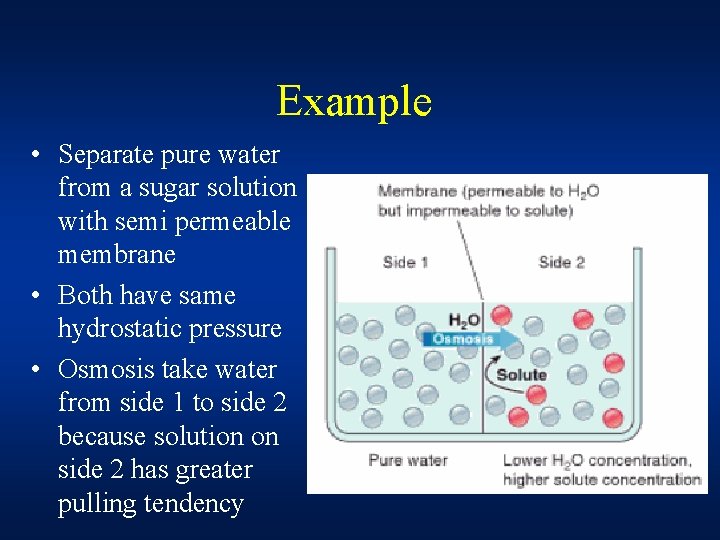
Example • Separate pure water from a sugar solution with semi permeable membrane • Both have same hydrostatic pressure • Osmosis take water from side 1 to side 2 because solution on side 2 has greater pulling tendency

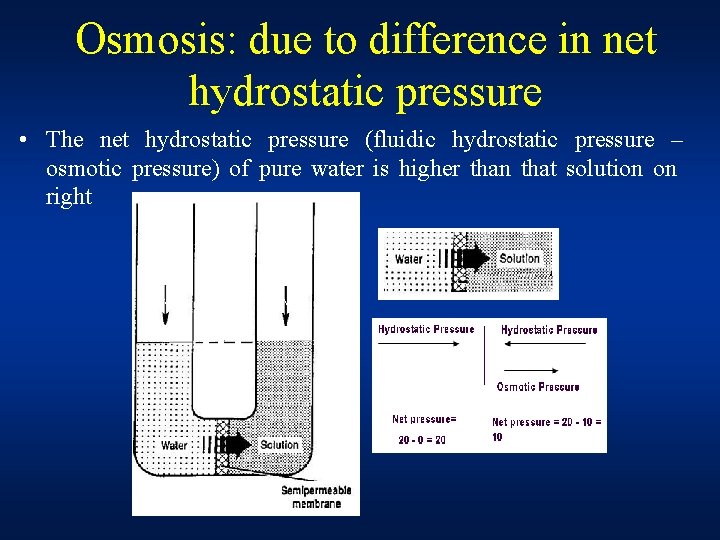
Osmosis: due to difference in net hydrostatic pressure • The net hydrostatic pressure (fluidic hydrostatic pressure – osmotic pressure) of pure water is higher than that solution on right

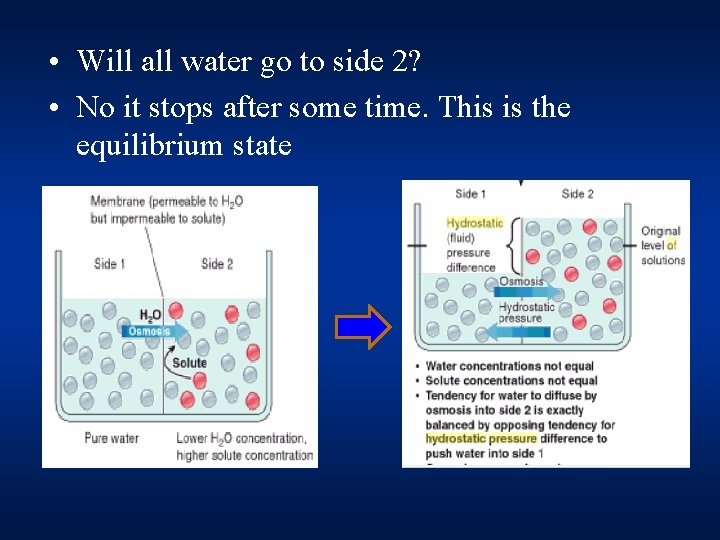
• Will all water go to side 2? • No it stops after some time. This is the equilibrium state

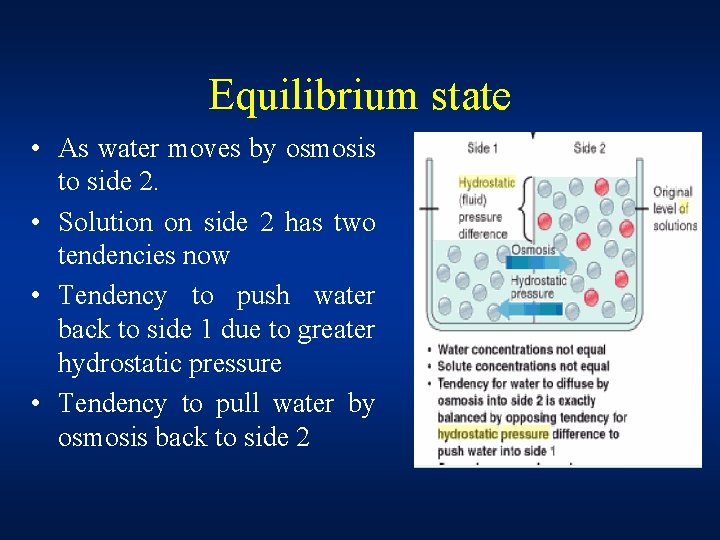
Equilibrium state • As water moves by osmosis to side 2. • Solution on side 2 has two tendencies now • Tendency to push water back to side 1 due to greater hydrostatic pressure • Tendency to pull water by osmosis back to side 2

Equilibrium state • Net movement of water by osmosis continues until the opposing hydrostatic pressure (a pushing pressure) exactly equals the osmotic pressure SO a common definition for osmotic pressure is • Osmosis may be opposed by increasing the pressure in the region of high solute concentration (hypertonic solution) with respect to that in the low solute concentration region (hypotonic solution). The hydrostatic pressure which just stops osmosis is the osmotic pressure • Osmotic pressure is the excess of the pressure required to equalize water activities in the two compartments • The term osmotic pressure is used for a solution and not for a solvent

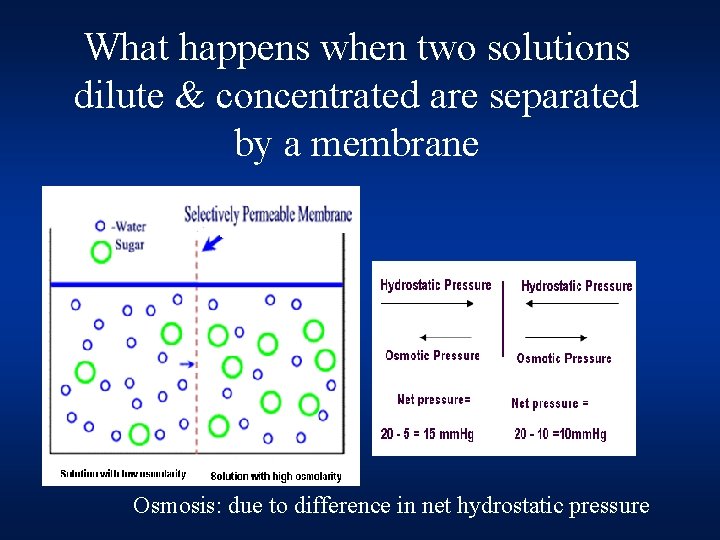
What happens when two solutions dilute & concentrated are separated by a membrane Osmosis: due to difference in net hydrostatic pressure

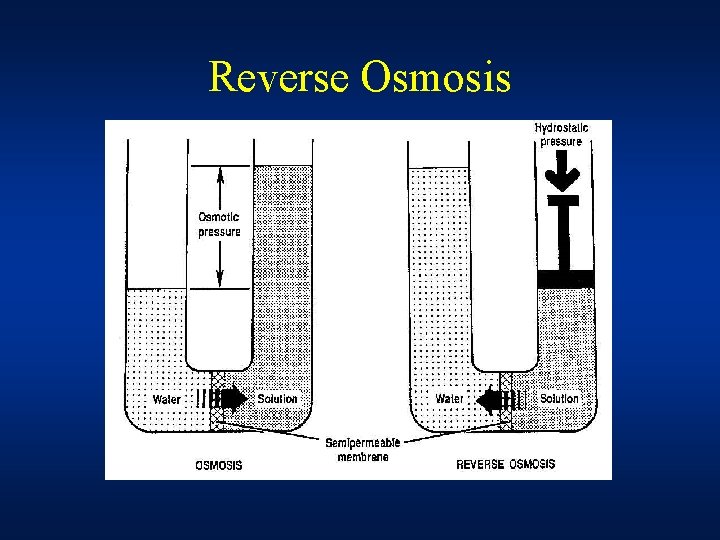
Reverse Osmosis • Reverse osmosis is a membrane based filtration method that removes many types of large molecules and ions from solutions by applying pressure to the solution when it is on one side of a selective membrane. • If an external pressure is applied on a concentrated solution, this pressure is distributed evenly throughout the solution • If the applied pressure is higher than the osmotic pressure water will flow towards the other side of the membrane leaving solute behind • This technique is used for purification of water

Reverse Osmosis

Importance of Osmosis and Osmotic Pressure • Oncotic pressure of blood plasma • Formation of tissue fluid • Regulation of cell volume

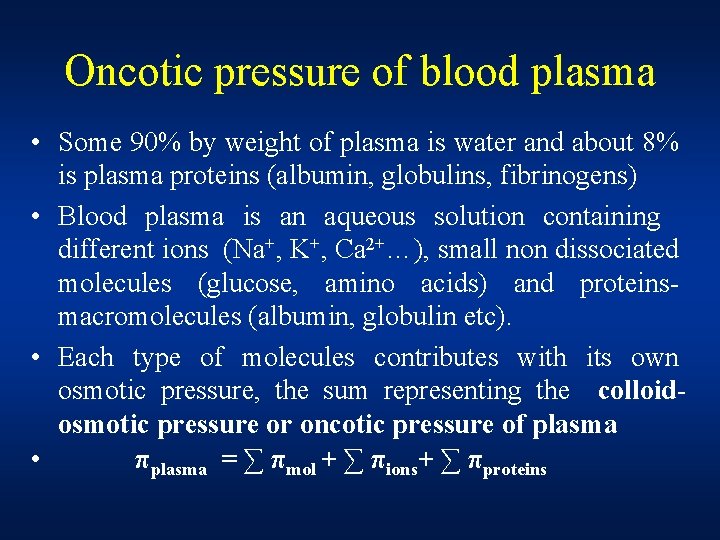
Oncotic pressure of blood plasma • Some 90% by weight of plasma is water and about 8% is plasma proteins (albumin, globulins, fibrinogens) • Blood plasma is an aqueous solution containing different ions (Na+, K+, Ca 2+…), small non dissociated molecules (glucose, amino acids) and proteinsmacromolecules (albumin, globulin etc). • Each type of molecules contributes with its own osmotic pressure, the sum representing the colloidosmotic pressure or oncotic pressure of plasma • πplasma = ∑ πmol + ∑ πions+ ∑ πproteins

Oncotic pressure of blood plasma • Oncotic pressure of plasma usually tends to pull water into the circulatory system • Albumin is the major contributor to oncotic pressure of plasma because it has the lowest molecular weight of the major plasma proteins and its concentration is almost double that of globulin • The total oncotic pressure of an average capillary is about 28 mm. Hg with albumin contributing approximately 22 mm. Hg of this oncotic pressure.

• Throughout the body, dissolved compounds have an osmotic pressure. Because large plasma proteins cannot easily cross through the capillary walls, their effect on the osmotic pressure of the capillary interiors will, to some extent, balance out the tendency for fluid to leak out of the capillaries. In other words, the oncotic pressure tends to pull fluid into the capillaries.

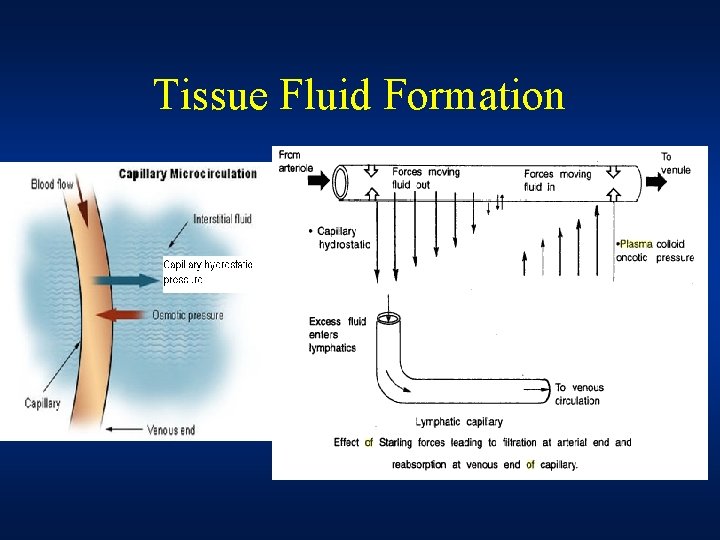
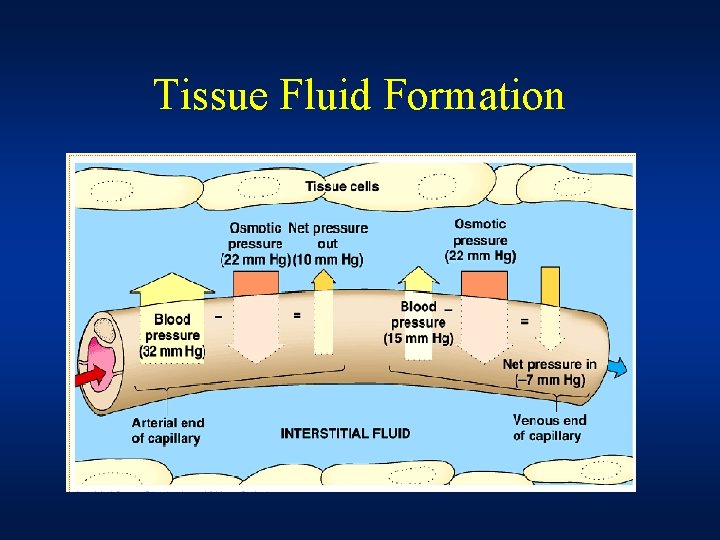
Tissue Fluid Formation • Filtration takes place at the arterial end of capillary because hydrostatic pressure of blood overcomes the oncotic pressure of plasma proteins • Reabsorption takes place at the venous end of capillary because hydrostatic pressure of blood falls below the oncotic pressure of plasma proteins • Net result of this filtration and reabsorption process is the tissue fluid formation.

• The balancing of filtration/ultrafiltration and reabsorption/osmosis at arterial and venous end of capillary is referred to as the Starling Equilibrium • Removal of tissue fluid To prevent a build up of tissue fluid surrounding the cells in the tissue, the lymphatic system plays a part in the transport of tissue fluid. Tissue fluid can pass into the surrounding lymph vessels, and eventually ends up rejoining the blood

Tissue Fluid Formation

Tissue Fluid Formation

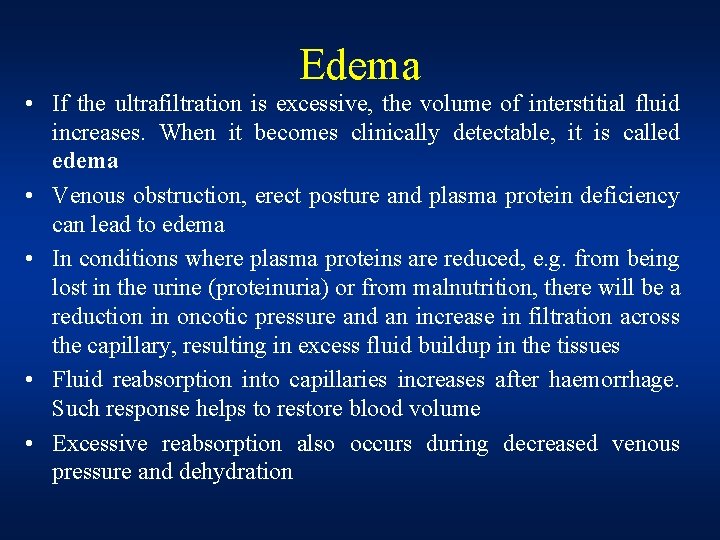
Edema • If the ultrafiltration is excessive, the volume of interstitial fluid increases. When it becomes clinically detectable, it is called edema • Venous obstruction, erect posture and plasma protein deficiency can lead to edema • In conditions where plasma proteins are reduced, e. g. from being lost in the urine (proteinuria) or from malnutrition, there will be a reduction in oncotic pressure and an increase in filtration across the capillary, resulting in excess fluid buildup in the tissues • Fluid reabsorption into capillaries increases after haemorrhage. Such response helps to restore blood volume • Excessive reabsorption also occurs during decreased venous pressure and dehydration

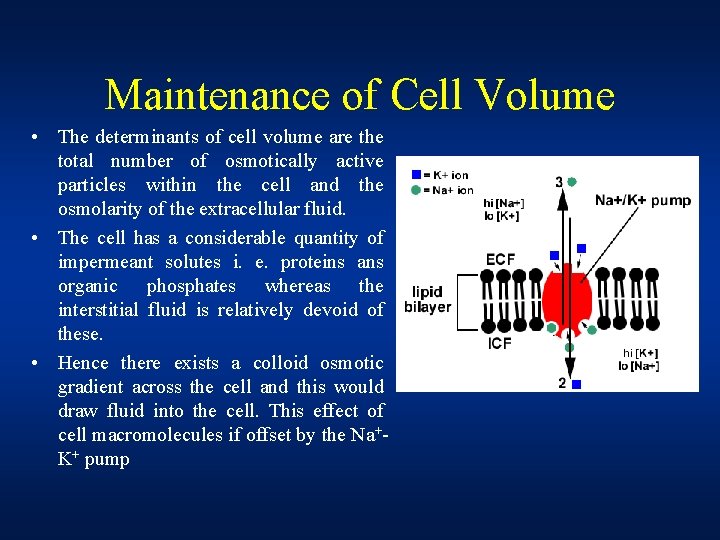
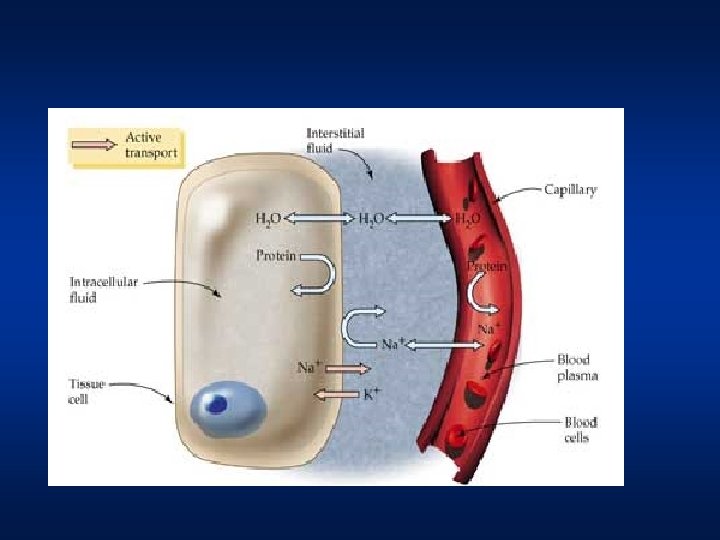
Maintenance of Cell Volume • The determinants of cell volume are the total number of osmotically active particles within the cell and the osmolarity of the extracellular fluid. • The cell has a considerable quantity of impermeant solutes i. e. proteins ans organic phosphates whereas the interstitial fluid is relatively devoid of these. • Hence there exists a colloid osmotic gradient across the cell and this would draw fluid into the cell. This effect of cell macromolecules if offset by the Na+K+ pump

Maintenance of Cell Volume • 3 positive ions (Na+) are pumped out of the cell (towards ECF) for every 2 positive ions (K+) pumped into the cell (towards ICF). This means that there is more positive charges leaving the cell than entering it. • As a result, positive charge builds up outside the cell compared to inside the cell. The difference in charge between the outside and inside of the cell limits the fluid flow into the cell • About 90% of the osmotic pressre of extracellular fluid is due to sodium ions


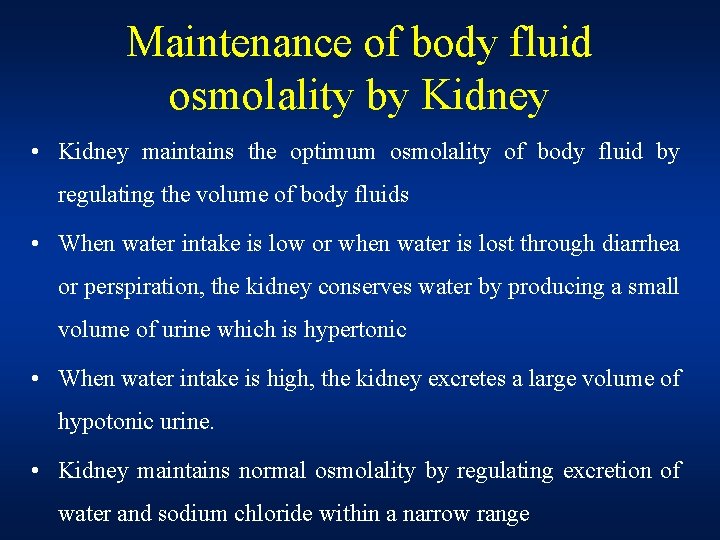
Maintenance of body fluid osmolality by Kidney • Kidney maintains the optimum osmolality of body fluid by regulating the volume of body fluids • When water intake is low or when water is lost through diarrhea or perspiration, the kidney conserves water by producing a small volume of urine which is hypertonic • When water intake is high, the kidney excretes a large volume of hypotonic urine. • Kidney maintains normal osmolality by regulating excretion of water and sodium chloride within a narrow range

Surface Tension • The force with which surface molecules are held is called the surface tension of the liquid • It is the force acting perpendicularly inward on the surface layer of a liquid to pull its surface molecules towards the interior of the fluid • It keeps the surface like a stretched membrane, and hence keeps the contact area minimum

Surface Tension • Water striders use surface tension to walk on the surface of pond. The surface of the water behaves like an elastic film: the insect's feet cause indentations in the water's surface. Its tiny mass and geometry of its legs allow it to be supported by the high surface tension of water • Formation of drops occurs when a mass of liquid is stretched. Water adhering to the tap gains mass until it is stretched to a point where the surface tension can no longer bind it to the tap. It then separates and surface tension forms the drop into a sphere. If a stream of water were running from the tap, the stream would break up into drops during its fall. Gravity stretches the stream, then surface tension pinches it into spheres

Surface tension at interfaces • • • Surface tension at liquid-air interface: A soap bubble is a thin film of soapy water enclosing air that forms a hollow sphere. Surface tension causes a bubble to assume the smallest surface area to contain a given volume -- resulting in the spherical shape Liquid-solid interface Beading of rain water on the surface of a waxy surface, such as a leaf. Water adheres weakly to wax and strongly to itself, so water clusters into drops. Surface tension gives them their near-spherical shape, because a sphere has the smallest possible surface area to volume ratio Liquid-Liquid interface Separation of oil and water (in this case, water and liquid wax) is caused by a tension in the surface between dissimilar liquids.

Factors affecting surface tension • Temperature Surface tension falls with rise in temperature hence increasing the surface area Major reason for using hot water for washing is that its surface tension is lower and it is a better wetting agent • Solutes can have different effects on surface tension depending on their structure: Little or no effect, for example sugar Increase surface tension, inorganic salts Decrease surface tension progressively, alcohols Decrease surface tension and, once a minimum is reached, no more effect: surfactants

Gibbs-Thomsan Principle • According to this principle substances which lower the surface tension becomes concentrated in the surface layer whereas substances which increase surface tension are distributed in the interior of the liquid • Lipids and proteins effective in lowering surface tension are found concentrated in the cell wall • Soaps and bile salts reduce the surface tension of water while sodium chloride and most inorganic salts increase the surface tension
 Osmolarity versus osmolality
Osmolarity versus osmolality Oncotic pressure
Oncotic pressure Interstitial fluid hydrostatic pressure
Interstitial fluid hydrostatic pressure Osmotic pressure vs hydrostatic
Osmotic pressure vs hydrostatic What is a colloid fluid
What is a colloid fluid Electrolytes and nonelectrolytes
Electrolytes and nonelectrolytes Osmosis equation
Osmosis equation Vapour pressure lowering
Vapour pressure lowering Characteristics of solutions
Characteristics of solutions Raoult's law
Raoult's law Equilibria
Equilibria Fluid dynamics
Fluid dynamics Dr hassan shah
Dr hassan shah Importance of osmosis
Importance of osmosis 01:640:244 lecture notes - lecture 15: plat, idah, farad
01:640:244 lecture notes - lecture 15: plat, idah, farad Bernoulli's principle pipe flow
Bernoulli's principle pipe flow High pressure and low pressure
High pressure and low pressure High pressure area
High pressure area How to find pressure potential
How to find pressure potential Osmosis water and salt
Osmosis water and salt Diffusion and osmosis
Diffusion and osmosis Elmslie trillat
Elmslie trillat Pressure support vs pressure control
Pressure support vs pressure control Continuous bedside pressure mapping
Continuous bedside pressure mapping Intrapleural pressure
Intrapleural pressure How to find partial pressure from total pressure
How to find partial pressure from total pressure Tripod position breathing
Tripod position breathing Metamorphic
Metamorphic ütube
ütube Afferent and efferent arterioles
Afferent and efferent arterioles Oncotic pressure vs hydrostatic pressure
Oncotic pressure vs hydrostatic pressure Confining pressure vs directed pressure
Confining pressure vs directed pressure Mean arterial pressure from blood pressure
Mean arterial pressure from blood pressure How to find partical pressure
How to find partical pressure Ventilator pressure support
Ventilator pressure support