Partial PressureIntro Consider these boxes all the same
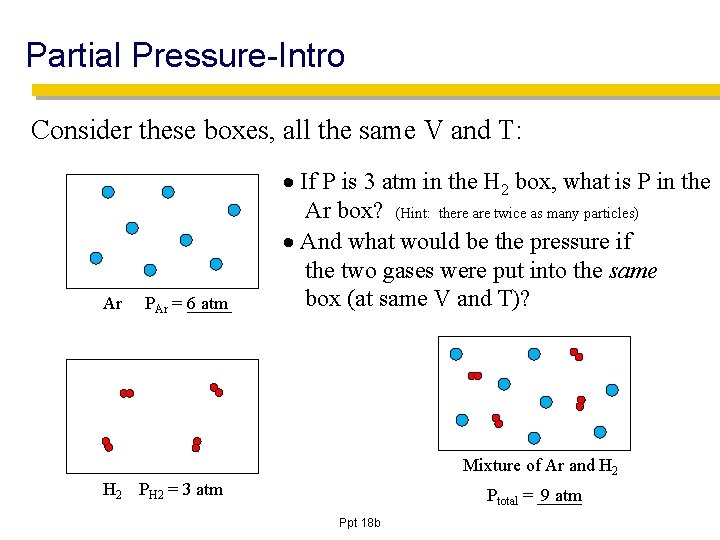
Partial Pressure-Intro Consider these boxes, all the same V and T: Ar PAr = _____ 6 atm If P is 3 atm in the H 2 box, what is P in the Ar box? (Hint: there are twice as many particles) And what would be the pressure if the two gases were put into the same box (at same V and T)? Mixture of Ar and H 2 PH 2 = 3 atm Ptotal = _____ 9 atm Ppt 18 b
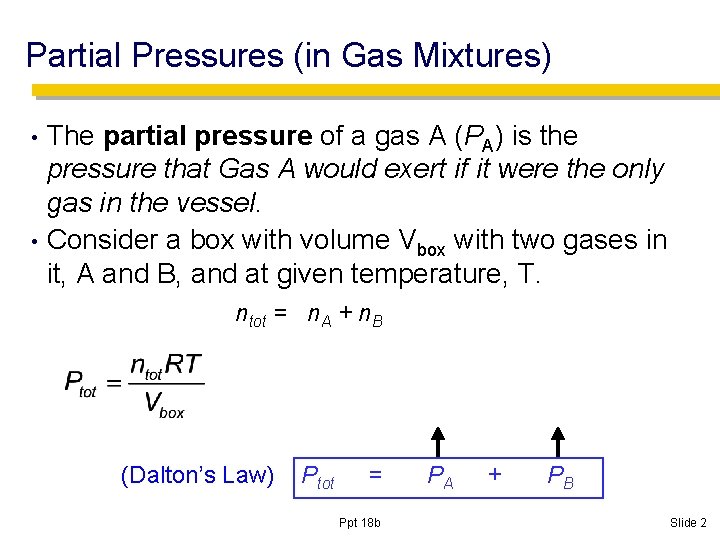
Partial Pressures (in Gas Mixtures) The partial pressure of a gas A (PA) is the pressure that Gas A would exert if it were the only gas in the vessel. • Consider a box with volume Vbox with two gases in it, A and B, and at given temperature, T. • ntot = n. A + n. B (Dalton’s Law) Ptot = Ppt 18 b PA + PB Slide 2
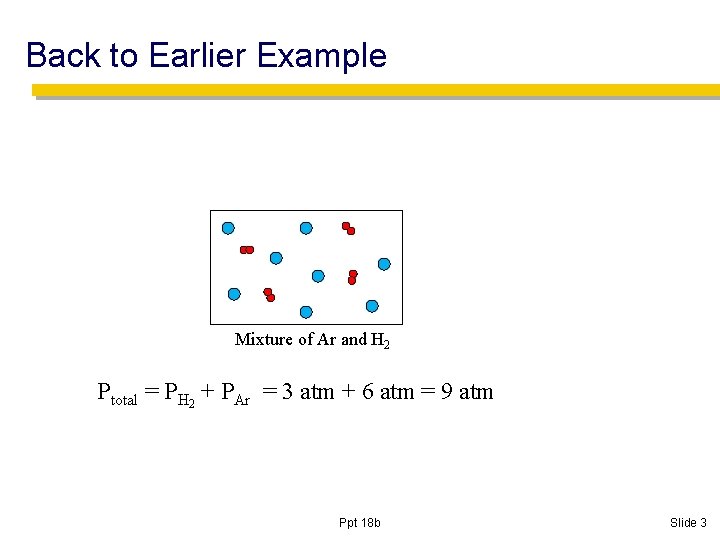
Back to Earlier Example Mixture of Ar and H 2 Ptotal = PH 2 + PAr = 3 atm + 6 atm = 9 atm Ppt 18 b Slide 3
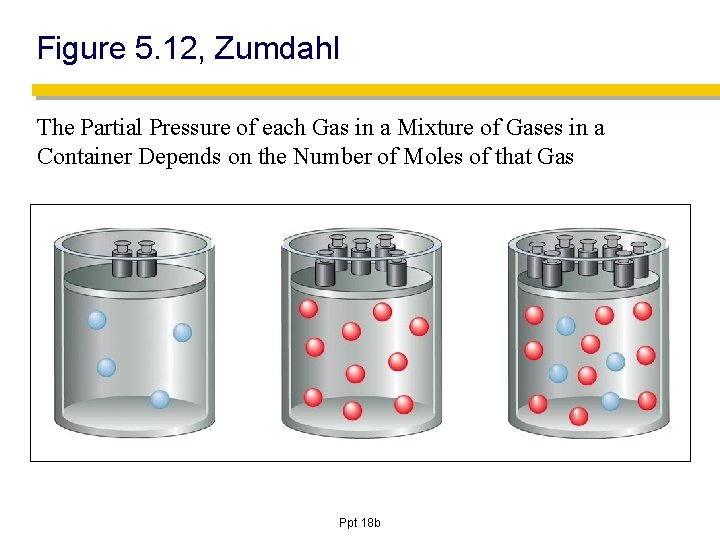
Figure 5. 12, Zumdahl The Partial Pressure of each Gas in a Mixture of Gases in a Container Depends on the Number of Moles of that Gas Ppt 18 b
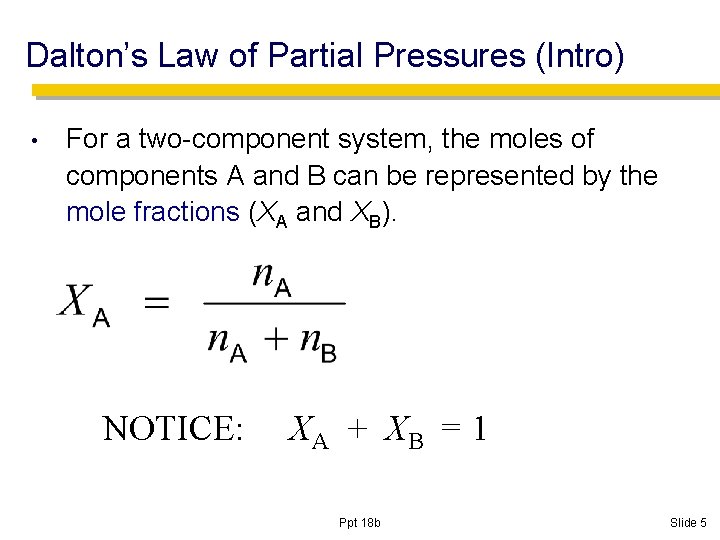
Dalton’s Law of Partial Pressures (Intro) • For a two-component system, the moles of components A and B can be represented by the mole fractions (XA and XB). NOTICE: XA + XB = 1 Ppt 18 b Slide 5
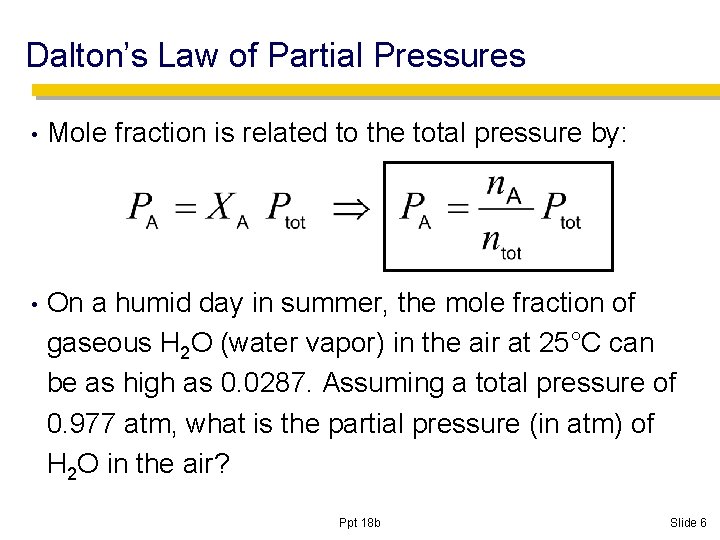
Dalton’s Law of Partial Pressures • Mole fraction is related to the total pressure by: • On a humid day in summer, the mole fraction of gaseous H 2 O (water vapor) in the air at 25°C can be as high as 0. 0287. Assuming a total pressure of 0. 977 atm, what is the partial pressure (in atm) of H 2 O in the air? Ppt 18 b Slide 6
Dalton’s Law of Partial Pressures • 2. 0 moles of Ne and 3. 0 moles of Ar were placed in a 40. 0 L container at 25 o. C. What are the partial pressures of each gas and the total pressure? Ppt 18 b Slide 7
Dalton’s Law of Partial Pressures • A sample of natural gas contains 6. 25 moles of methane (CH 4), 0. 500 moles of ethane (C 2 H 6), and 0. 100 moles of propane (C 3 H 8). If the total pressure of the gas is 1. 50 atm, what are the partial pressures of the gases? Ppt 18 b Slide 8
Dalton’s Law of Partial Pressures • Hydrogen gas generated when calcium metal reacts with water is collected at 30°C and a pressure of 988 mm Hg. The volume collected is 641 ml. What is the mass (in grams) of the hydrogen gas obtained? The pressure of the water vapor at 30°C is 32 mm Hg. • 760 mm Hg = 1. 00 atm Ppt 18 b Slide 9
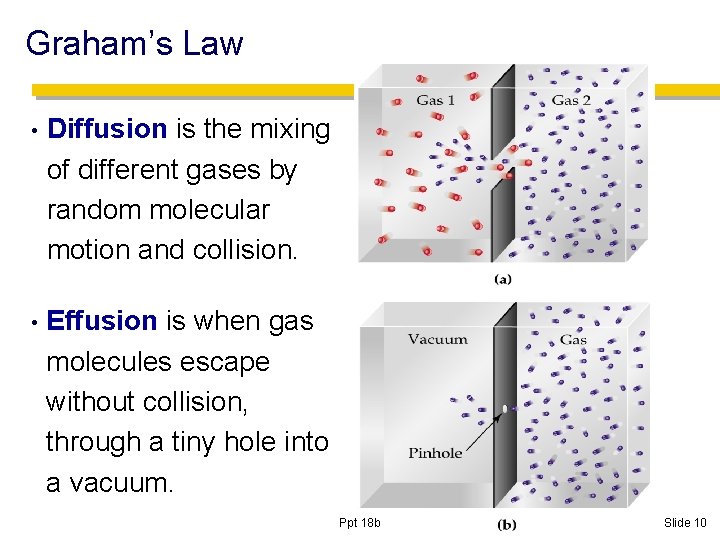
Graham’s Law • Diffusion is the mixing of different gases by random molecular motion and collision. • Effusion is when gas molecules escape without collision, through a tiny hole into a vacuum. Ppt 18 b Slide 10
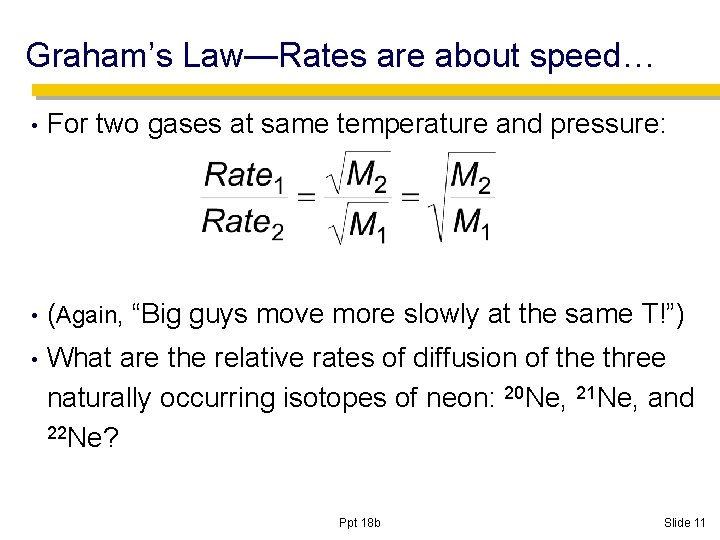
Graham’s Law—Rates are about speed… • For two gases at same temperature and pressure: • (Again, • “Big guys move more slowly at the same T!”) What are the relative rates of diffusion of the three naturally occurring isotopes of neon: 20 Ne, 21 Ne, and 22 Ne? Ppt 18 b Slide 11
- Slides: 11