Gases Manometers measure P of a gas 1




- Slides: 16

Gases

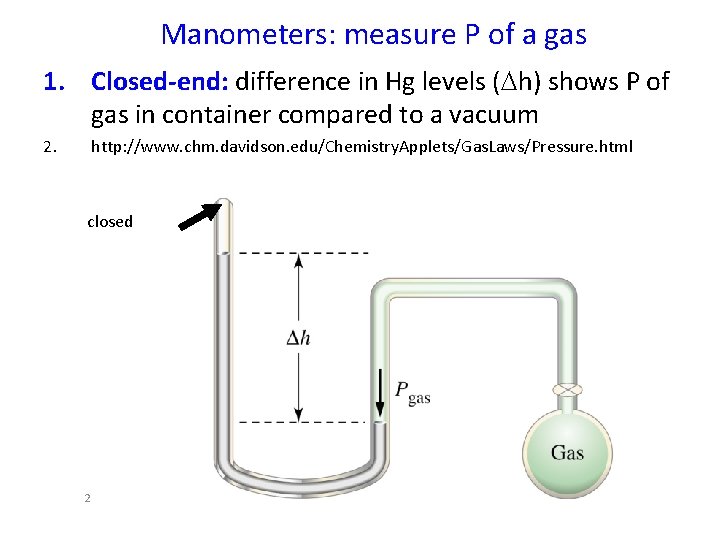
Manometers: measure P of a gas 1. Closed-end: difference in Hg levels (Dh) shows P of gas in container compared to a vacuum 2. http: //www. chm. davidson. edu/Chemistry. Applets/Gas. Laws/Pressure. html closed 2

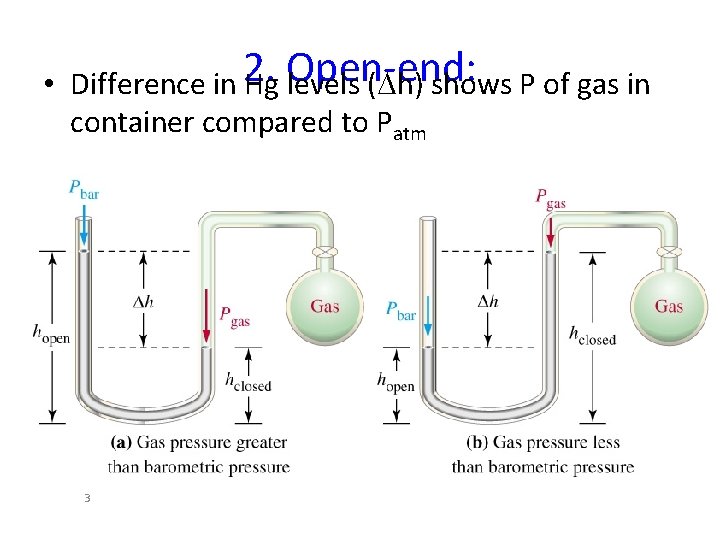
• Difference in 2. Hg Open-end: levels (Dh) shows P of gas in container compared to Patm 3

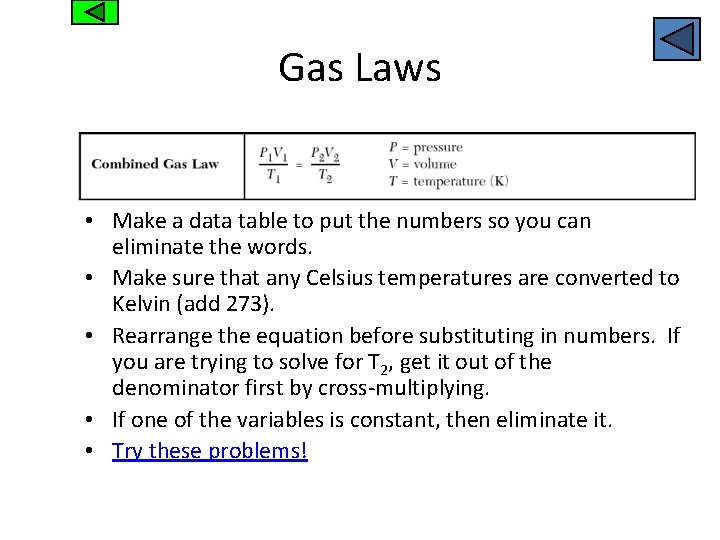
Gas Laws • Make a data table to put the numbers so you can eliminate the words. • Make sure that any Celsius temperatures are converted to Kelvin (add 273). • Rearrange the equation before substituting in numbers. If you are trying to solve for T 2, get it out of the denominator first by cross-multiplying. • If one of the variables is constant, then eliminate it. • Try these problems!

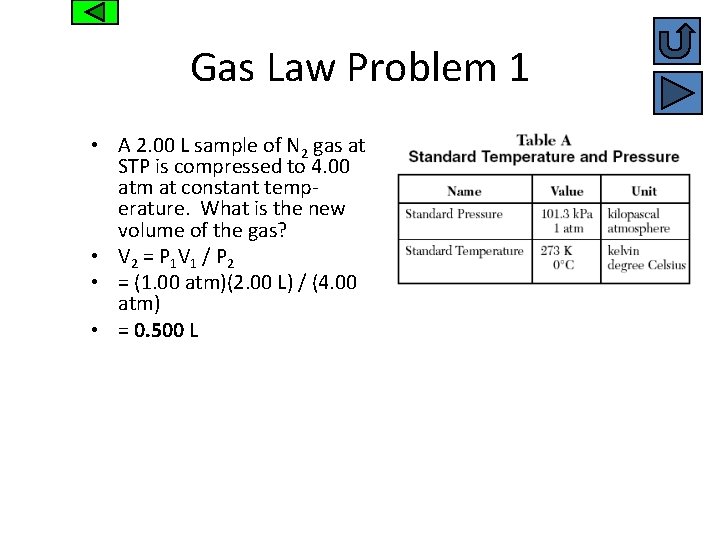
Gas Law Problem 1 • A 2. 00 L sample of N 2 gas at STP is compressed to 4. 00 atm at constant temperature. What is the new volume of the gas? • V 2 = P 1 V 1 / P 2 • = (1. 00 atm)(2. 00 L) / (4. 00 atm) • = 0. 500 L

Gas Law Problem 2 • To what temperature must a 3. 000 L sample of O 2 gas at 300. 0 K be heated to raise the volume to 10. 00 L? • T 2 = V 2 T 1/V 1 • = (10. 00 L)(300. 0 K) / (3. 000 L) = 1000. K

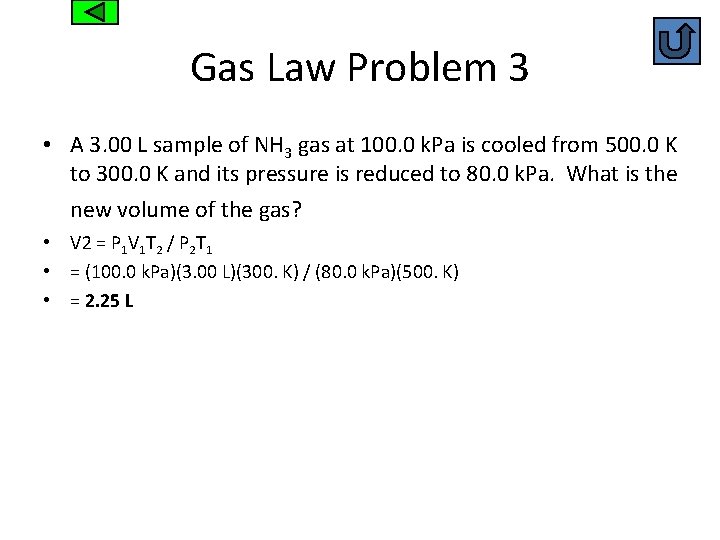
Gas Law Problem 3 • A 3. 00 L sample of NH 3 gas at 100. 0 k. Pa is cooled from 500. 0 K to 300. 0 K and its pressure is reduced to 80. 0 k. Pa. What is the new volume of the gas? • V 2 = P 1 V 1 T 2 / P 2 T 1 • = (100. 0 k. Pa)(3. 00 L)(300. K) / (80. 0 k. Pa)(500. K) • = 2. 25 L

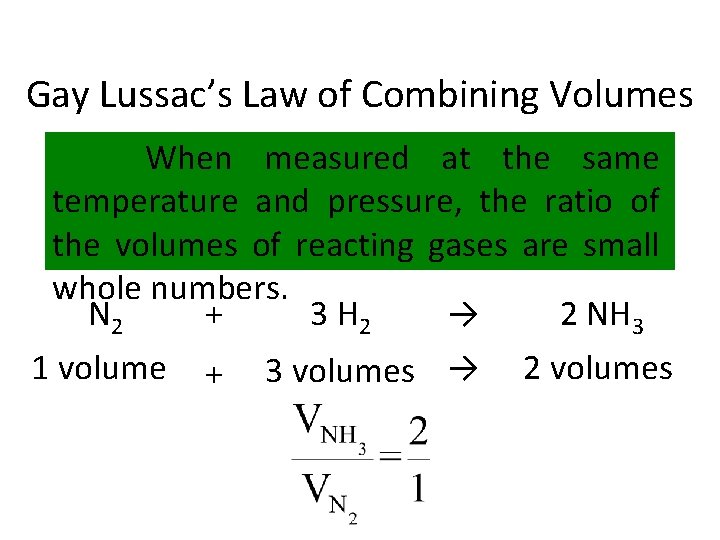
Gay Lussac’s Law of Combining Volumes When measured at the same temperature and pressure, the ratio of the volumes of reacting gases are small whole numbers. N 2 + 3 H 2 → 2 NH 3 1 volume + 3 volumes → 2 volumes

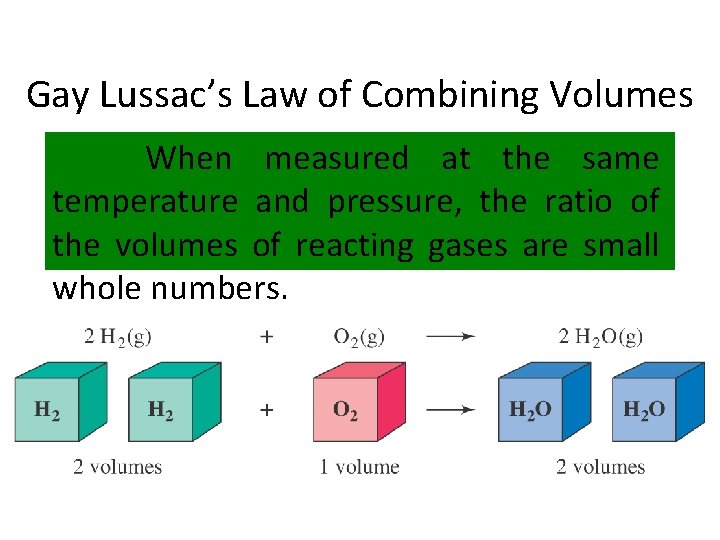
Gay Lussac’s Law of Combining Volumes When measured at the same temperature and pressure, the ratio of the volumes of reacting gases are small whole numbers.

Avogadro’s Law Equal volumes of different gases at the same temperature and pressure contain the same number of molecules.

Mole-Mass-Volume Relationships • Volume of one mole of any gas at STP = 22. 4 L. • 22. 4 L at STP is known as the molar volume of any gas.

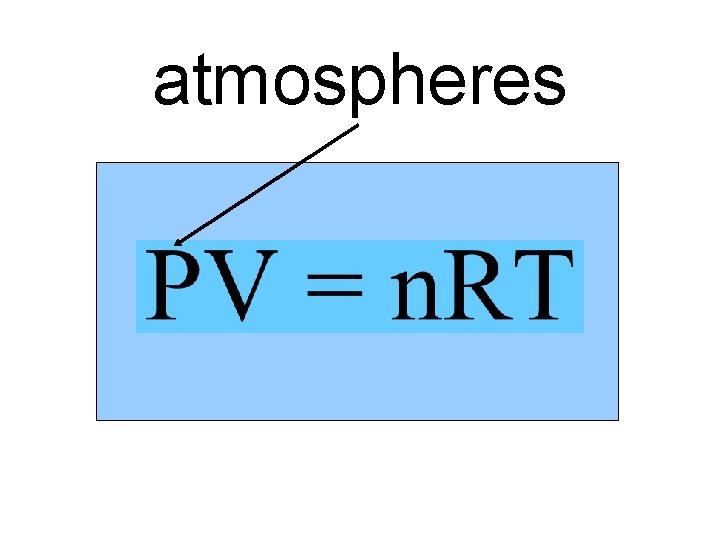
atmospheres n. T Va P

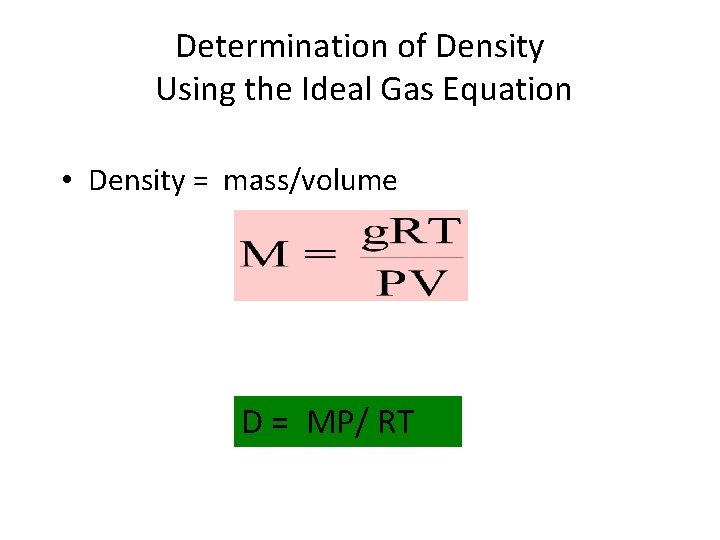
Determination of Density Using the Ideal Gas Equation • Density = mass/volume D = MP/ RT

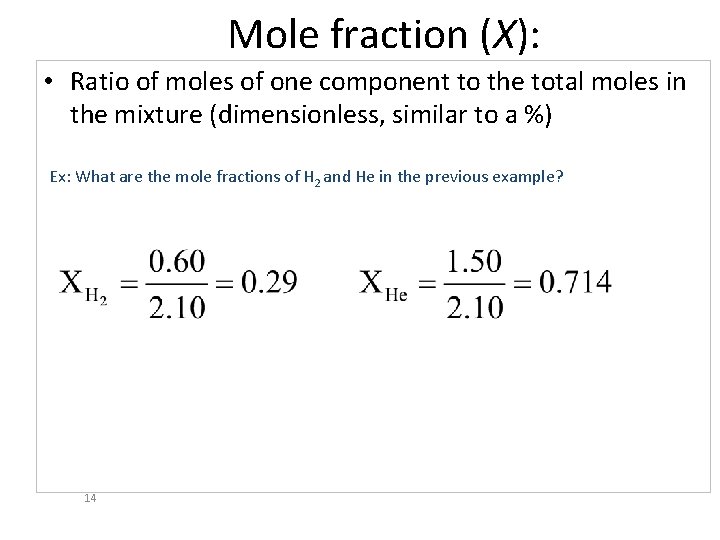
Mole fraction (X): • Ratio of moles of one component to the total moles in the mixture (dimensionless, similar to a %) Ex: What are the mole fractions of H 2 and He in the previous example? 14

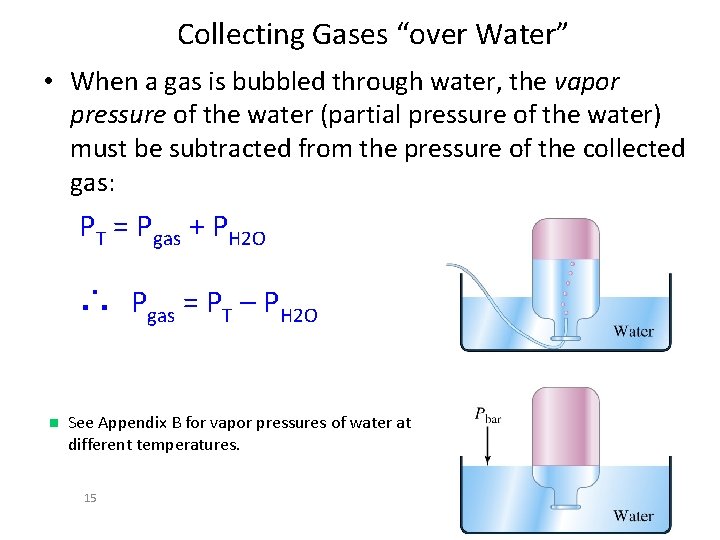
Collecting Gases “over Water” • When a gas is bubbled through water, the vapor pressure of the water (partial pressure of the water) must be subtracted from the pressure of the collected gas: PT = Pgas + PH 2 O ∴ Pgas = PT – PH 2 O n See Appendix B for vapor pressures of water at different temperatures. 15

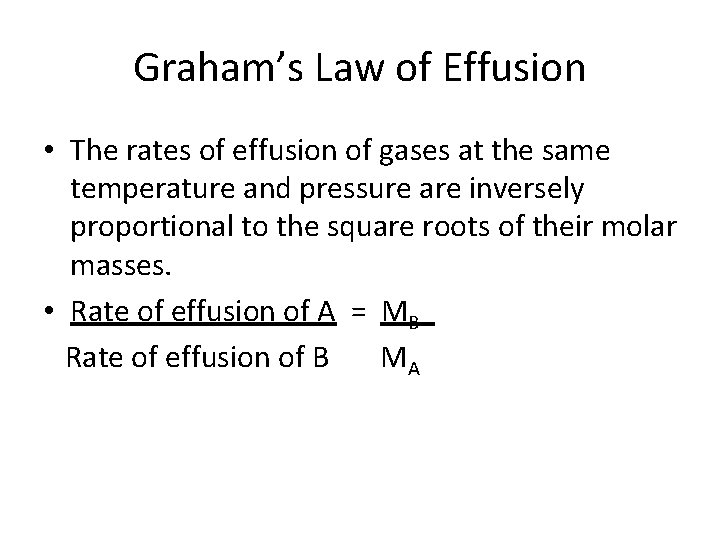
Graham’s Law of Effusion • The rates of effusion of gases at the same temperature and pressure are inversely proportional to the square roots of their molar masses. • Rate of effusion of A = MB Rate of effusion of B MA
 What weather instrument measures air temperature
What weather instrument measures air temperature Gibbons jacobean city comedy download
Gibbons jacobean city comedy download Volume molare
Volume molare Kinetika kimia
Kinetika kimia Ideal gas vs perfect gas
Ideal gas vs perfect gas Flue gas desulfurisation gas filter
Flue gas desulfurisation gas filter Ideal gas vs perfect gas
Ideal gas vs perfect gas Gas exchange key events in gas exchange
Gas exchange key events in gas exchange Conclusion of bhopal gas tragedy
Conclusion of bhopal gas tragedy Poisonous gas leaked in bhopal gas tragedy
Poisonous gas leaked in bhopal gas tragedy Imaginary gas
Imaginary gas Gas leaked in bhopal gas tragedy
Gas leaked in bhopal gas tragedy Difference between ideal gas and real gas
Difference between ideal gas and real gas Differences between ideal gas and real gas
Differences between ideal gas and real gas Gaseoso
Gaseoso Diagrama pv
Diagrama pv Diffusion of gases across respiratory membranes:
Diffusion of gases across respiratory membranes: