Unit 11 Gases Properties of Gases Gases expand












- Slides: 18

Unit 11: Gases: Properties of Gases

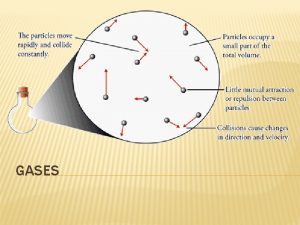
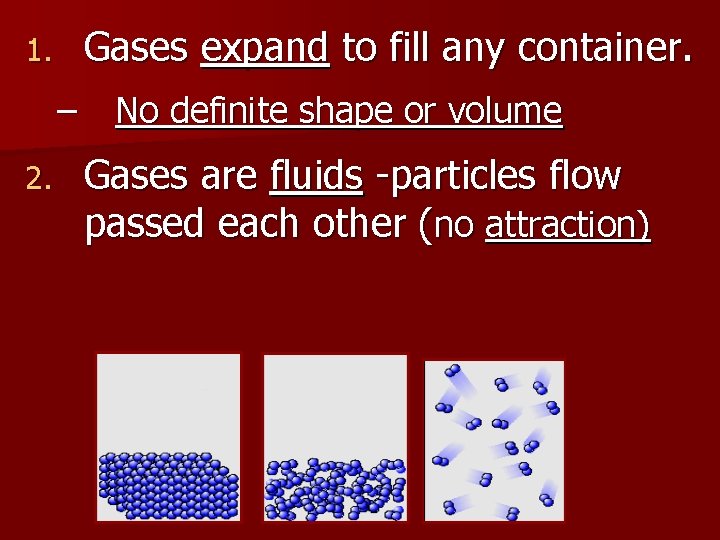
Gases expand to fill any container. 1. – 2. No definite shape or volume Gases are fluids -particles flow passed each other (no attraction)

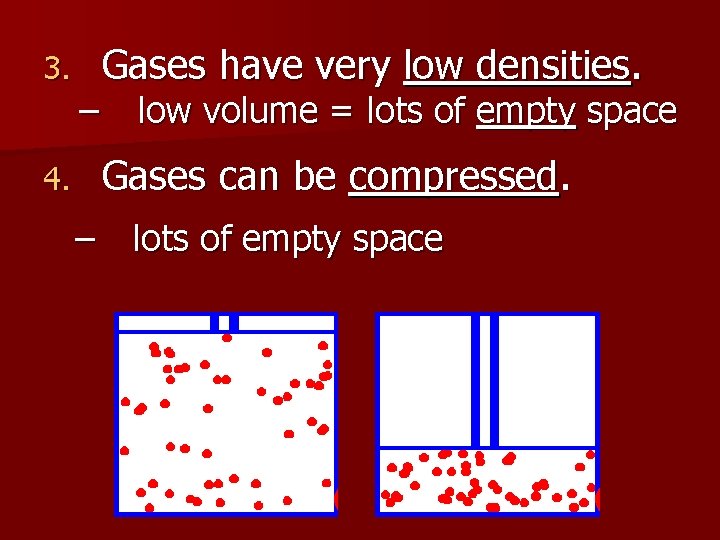
3. – Gases have very low densities. low volume = lots of empty space Gases can be compressed. 4. – lots of empty space

5. Gases undergo diffusion- spreading out of particles, mixing with other gases 6. Gases undergo effusion- gas particles pass through a tiny opening

Factors Affecting Gases

1. Volume (V) The amount space occupied by an object (gas) 3 • Units: L, m. L, cm •

2. Temperature (T) The amount of kinetic energy of the particles • Units: K •

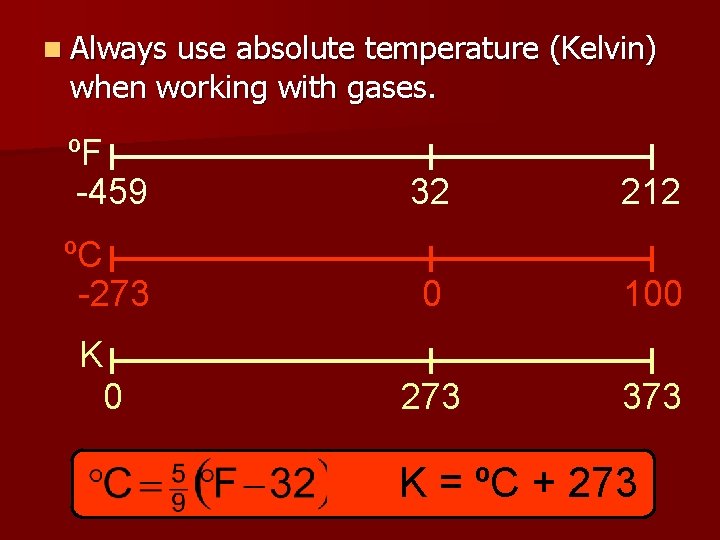
n Always use absolute temperature (Kelvin) when working with gases. ºF -459 32 212 ºC -273 0 100 K 0 273 373 K = ºC + 273

3. # of gas particles (n) • The amount moles in a gas • Units: mol

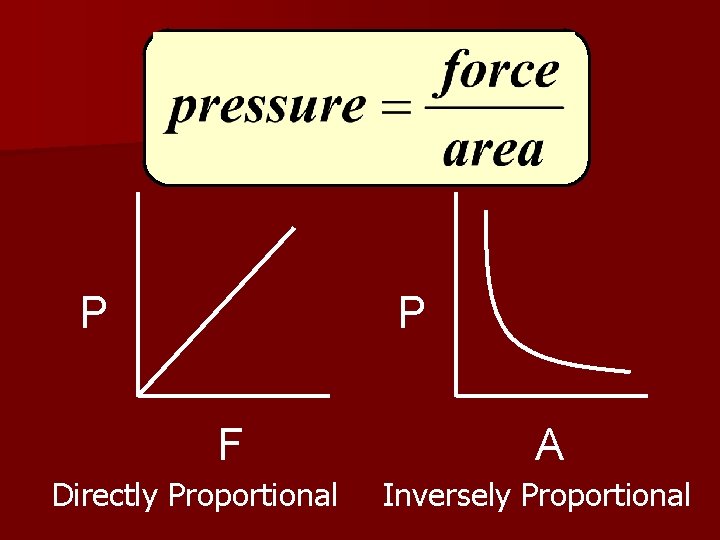
4. Pressure (Pa) The amount of force exerted over a given area.

P P F Directly Proportional A Inversely Proportional

Which shoes create the most pressure?

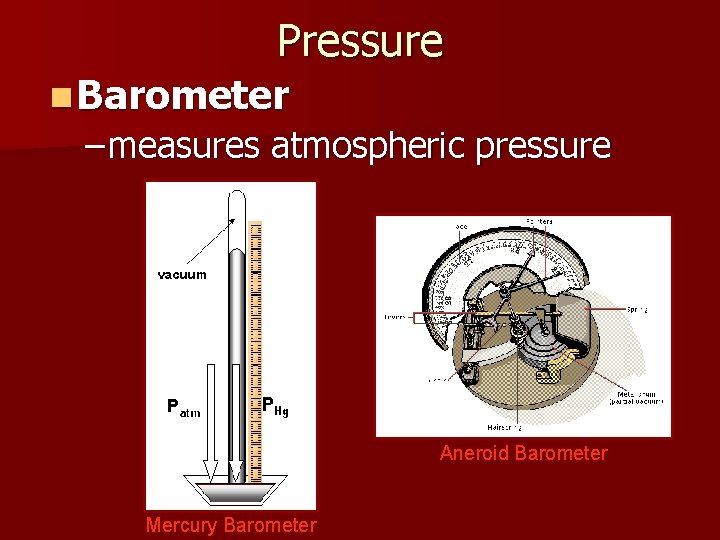
Pressure n Barometer – measures atmospheric pressure Aneroid Barometer Mercury Barometer

Units of pressure • atm (atmosphere) • psi (pounds per square inch) • torr • mm. Hg (millimeters of mercury) • Pa (Pascal) • k. Pa (kilo Pascal)

Conversion factors: 1 atm = 14. 7 psi = 760 torr = 760 mm. Hg = 1. 01325 x 105 Pa = 101. 325 k. Pa

Pressure Let’s do some practice conversions: 156. 1 k. Pa = ? psi Ans = 22. 65 psi

Pressure Let’s do some practice conversions: 689 torr = ? atm Ans = 0. 907 atm

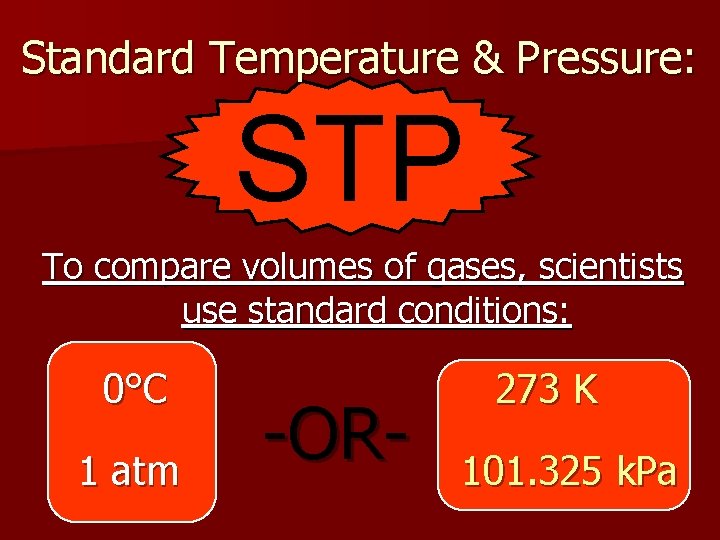
Standard Temperature & Pressure: STP To compare volumes of gases, scientists use standard conditions: 0°C 1 atm -OR- 273 K 101. 325 k. Pa
 Language
Language Buoyancyability
Buoyancyability 20 examples of liquids
20 examples of liquids What are the general properties of gases
What are the general properties of gases Four properties of gas
Four properties of gas 5 properties of gases
5 properties of gases Properties of gases
Properties of gases Characteristic of noble gas
Characteristic of noble gas What are the different properties of gas?
What are the different properties of gas? Properties of gases
Properties of gases Liperamid
Liperamid Properties of solid liquid and gas
Properties of solid liquid and gas State avogadro's law
State avogadro's law Four properties of gases
Four properties of gases List 2 of the important properties of gases
List 2 of the important properties of gases Unit 6 review questions
Unit 6 review questions Expanding the pie negotiation example
Expanding the pie negotiation example Expand igp in microfinance
Expand igp in microfinance Expand 3(2+t)
Expand 3(2+t)