Properties of Gases Kinetic Theory of Gases 1


























- Slides: 29

Properties of Gases

Kinetic Theory of Gases 1. Many independent particles 2. Random motion at high speed 3. Separated by great distances

Kinetic Theory of Gases 4. Interact only when they collide 5. 5. Elastic collisions

Physical Properties of Gases Diffusion Effusion Permeability Compressibility Expansibility

Diffusion spontaneous mixing due to particle motion

Effusion gas particles passing through a tiny opening into an evacuated area

Both diffusion and effusion are directly related to the speed of the gas molecules.

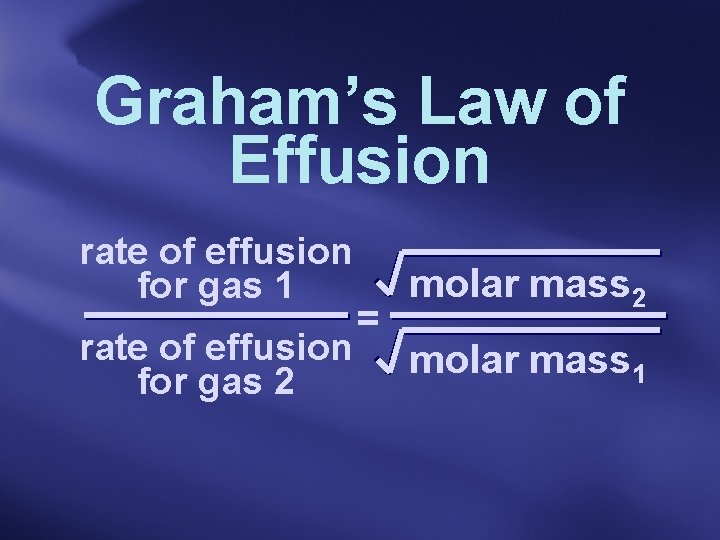
Graham’s Law of Effusion rate of effusion for gas 1 rate of effusion for gas 2 = molar mass 2 molar mass 1

Sample Problem 1 Calculate the ratio of effusion rates between nitrogen (N 2) and Argon (Ar). molar mass. N = 28. 02 g/mol molar mass. Ar = 39. 95 g/mol

Sample Problem 1 rate of effusion forgas N 2 1 rate of effusion forgas Ar 2 = molar 39. 95 mass g/mol 2 molar 28. 02 mass g/mol 1 = 1. 194

Sample Problem 1 The result indicates that the lighter nitrogen gas will effuse 1. 194 times faster than the argon gas.

Permeability the property of a substance that allows another substance’s particles to spread or flow throughout it

Compressibility the property of a substance that allows its particles to be squeezed into smaller volumes

Expansibility the property of a substance that allows its particles to spread out Expansibility is caused by pressure differences.

Question Why can gases expand so much? 1. 2. 3. 4. 5. Random motion Elastic collisions Independent particles Touching particles Particles locked together

Question Why can gases contract so much? 1. Separation of particles by great distances 2. Random motion 3. Particle interaction only when they collide 4. Elastic collisions 5. They cannot contract.

Gas Pressure average force per unit area

collisions

motion

Question What is the root cause of pressure? 1. Collisions 2. Motion 3. Force 4. Permeability

Units of Pressure 1 atmosphere (atm) = 14. 7 lb. /in 2 (psi) = 760 torr = 760 mm Hg – a standard unit of pressure used in barometers

Units of Pressure pascals (Pa) – the SI unit of pressure 1 atm = 101, 325 Pa = 101. 3 k. Pa

Barometer an apparatus that measures atmospheric pressure by its support of a column of liquid

Atmosphere Earth’s normal atmospheric pressure at sea level is 1 atm. (1 atm = 760 mm Hg)

Sample Problem 2 If a submarine is under a pressure of 17 atm, what is this pressure in mm Hg? 17 atm 760 mm Hg = 12, 920 1 atm mm Hg

Question If a bird is under a pressure of 800 torr, what is the pressure in atm? 1. 0. 94 atm 2. 54. 42 atm 3. 608, 000 atm 4. 1. 05 atm

Question If a bird is under a pressure of 800 torr, what is the pressure in atm? 800 torr 1 atm = 1. 05 760 torr atm

Pressure, Volume, and Temperature These three conditions of a gas are interrelated.

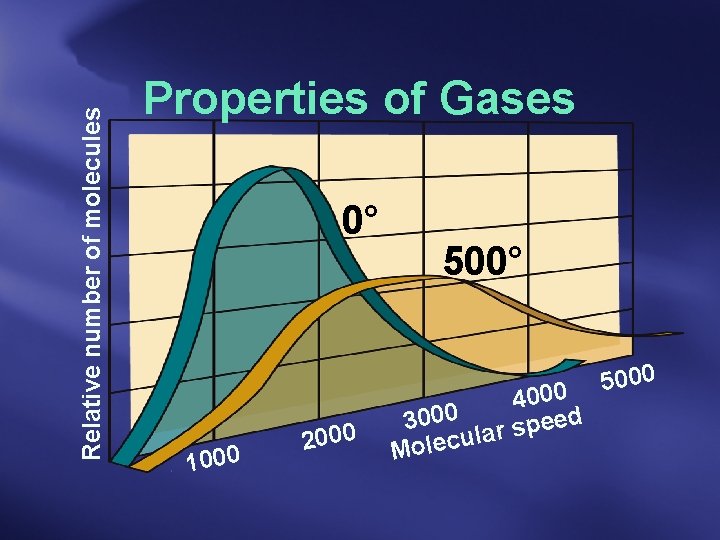
Relative number of molecules Properties of Gases 0° 1000 2000 500° 4000 3000 r speed a l u c e l Mo 5000
 Kinetic molecular theory of gases
Kinetic molecular theory of gases Particle theory of freezing
Particle theory of freezing Kinetic theory of gases
Kinetic theory of gases Three postulates of kinetic theory of gases
Three postulates of kinetic theory of gases Kinetic theory
Kinetic theory Postulates of kinetic theory of gases
Postulates of kinetic theory of gases Write the postulates of kinetic theory of gases
Write the postulates of kinetic theory of gases Kinetic theory of gases
Kinetic theory of gases Types of colloids
Types of colloids Tyndall effect
Tyndall effect Solids liquids and gases section 2 properties of fluids
Solids liquids and gases section 2 properties of fluids Solid liquid and gas particles
Solid liquid and gas particles What are the general properties of gases
What are the general properties of gases Four properties of gas
Four properties of gas 5 properties of gases
5 properties of gases Properties of gases
Properties of gases Characteristic of noble gas
Characteristic of noble gas Characteristics of gases
Characteristics of gases Gases have low densities
Gases have low densities Chemical properties of noble gases
Chemical properties of noble gases Liquid information
Liquid information Properties of gasses
Properties of gasses Properties of gases
Properties of gases List 2 of the important properties of gases
List 2 of the important properties of gases Kinetic molecular theory of solids
Kinetic molecular theory of solids The kinetic theory of matter states that
The kinetic theory of matter states that Kinetic theory of matter definition
Kinetic theory of matter definition Kinetic theory of matter
Kinetic theory of matter An explanation of how particles in matter behave
An explanation of how particles in matter behave The kinetic molecular theory
The kinetic molecular theory