GASES Gas Properties Four properties determine the physical
















- Slides: 27

GASES

Gas Properties Four properties determine the physical behavior of any gas: Amount of gas Gas pressure Gas volume Gas temperature 2

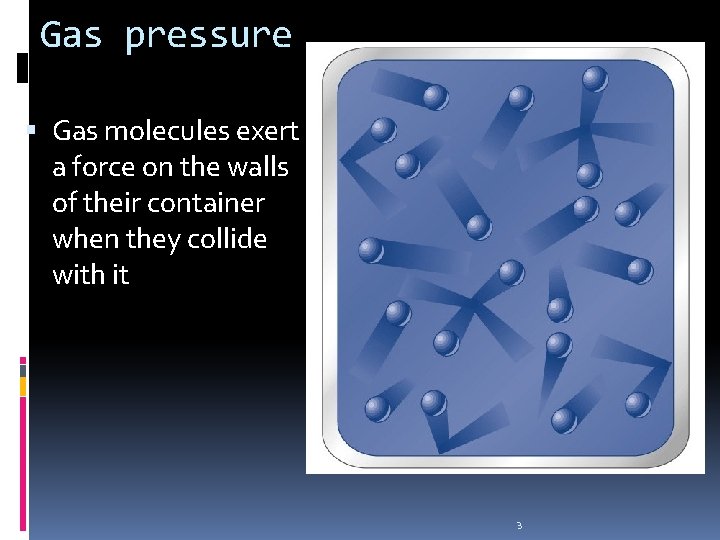
Gas pressure Gas molecules exert a force on the walls of their container when they collide with it 3

Atmospheric pressure Torricelli barometer In the closed tube, the liquid falls until the pressure exerted by the column of liquid just balances the pressure exerted by the atmosphere. Patmosphere proportional to height of liquid in tube Standard atmospheric pressure (1 atm) is 760 mm Hg 4

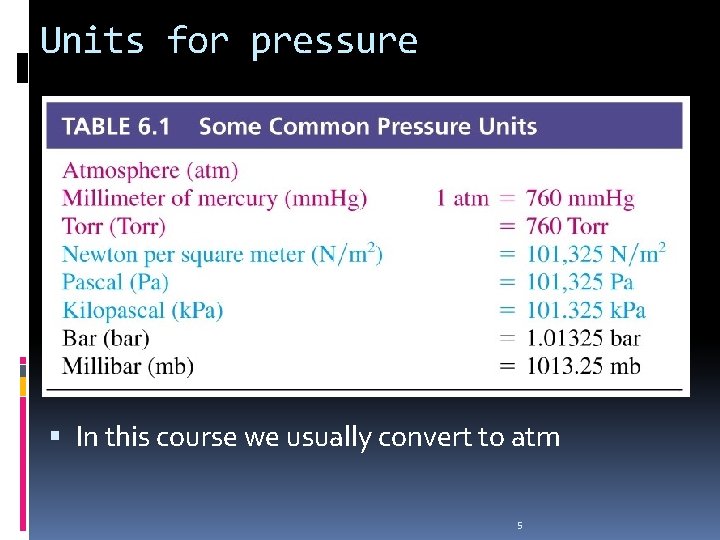
Units for pressure In this course we usually convert to atm 5

Let’s practice… Standard atmospheric pressure: 1 atm = 760 mm. Hg Convert 625 mm. Hg into atm Convert 2. 5 atm into mm. Hg

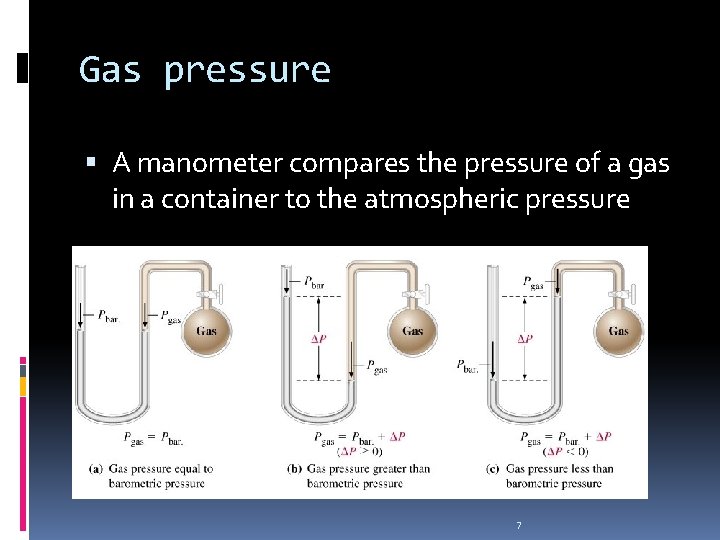
Gas pressure A manometer compares the pressure of a gas in a container to the atmospheric pressure 7

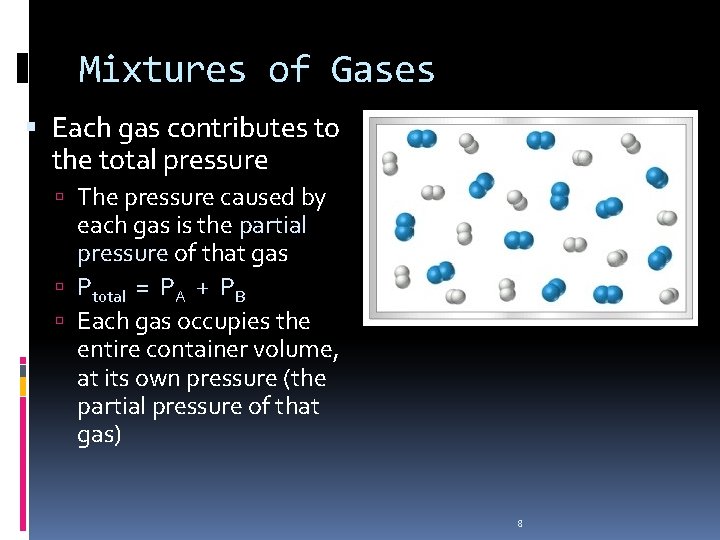
Mixtures of Gases Each gas contributes to the total pressure The pressure caused by each gas is the partial pressure of that gas Ptotal = PA + PB Each gas occupies the entire container volume, at its own pressure (the partial pressure of that gas) 8

Mixtures of Gases When a gas is collected over water, it is always “wet” (mixed with water vapor). Ptotal = Pbarometric = Pgas + Pwater vapor Example: If 35. 5 m. L of H 2 are collected over water at 26 °C and a barometric pressure of 755 mm Hg, what is the pressure of the H 2 gas? The water vapor pressure at 26 °C is 25. 2 mm Hg. 9

Relationships between gas properties: pressure, volume, and temp 1660 Robert Boyle investigates P and V: Indirect Relationship: Pressure Increases, Volume Decrease Pressure Decreases, Volume Increases PV = constant or P 1 V 1 = P 2 V 2

Let’s Practice… A sample of gas occupies 10 L at. 800 atm. What will the volume be if the pressure decreases to. 750 atm? A sample of gas occupies 25 L at 1. 5 atm. What will the new pressure be, if the volume increases to 30 L?

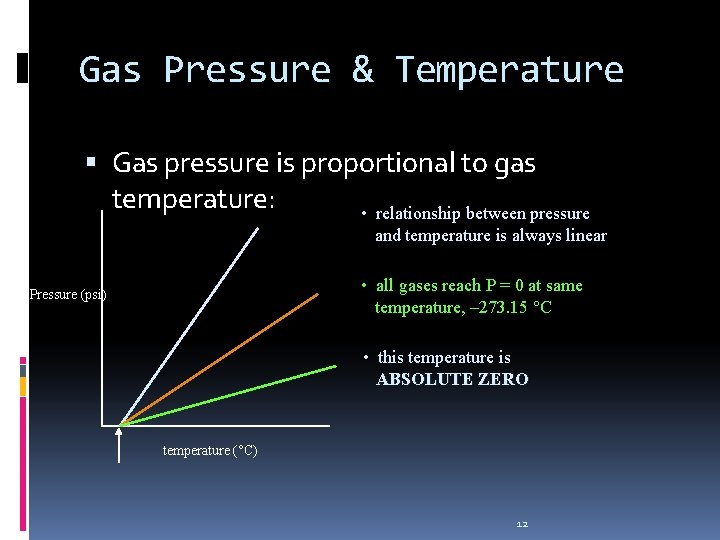
Gas Pressure & Temperature Gas pressure is proportional to gas temperature: • relationship between pressure and temperature is always linear • all gases reach P = 0 at same temperature, – 273. 15 °C Pressure (psi) • this temperature is ABSOLUTE ZERO temperature (°C) 12

Let’s practice…

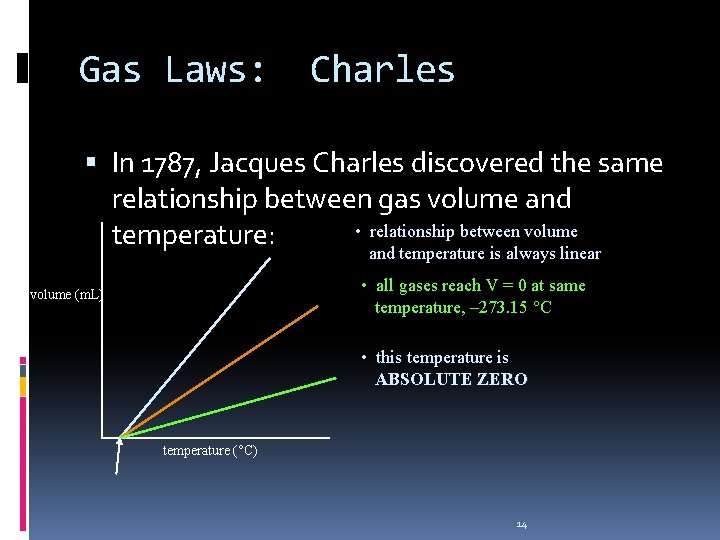
Gas Laws: Charles In 1787, Jacques Charles discovered the same relationship between gas volume and • relationship between volume temperature: and temperature is always linear • all gases reach V = 0 at same temperature, – 273. 15 °C volume (m. L) • this temperature is ABSOLUTE ZERO temperature (°C) 14

Let’s Practice…

A temperature scale for gases: the Kelvin scale 1860 English physicist, William Thomson (Lord Kelvin), suggests a relationship between kinetic energy and temperature. A new temperature scale was invented that has zero = absolute zero The new temperature scale was named the Kelvin or absolute temperature scale K = °C + 273. 15 16

Let’s Practice… K = °C + 273. 15 Convert 98. 6 °C into Kelvin Convert 125 K into °C

Temperature and Kinetic Energy The absolute (Kelvin) temperature of a substance is directly proportional to the kinetic energy of its molecules. Kinetic energy is the energy an object has because of its motion. KE = 1/2 mv 2

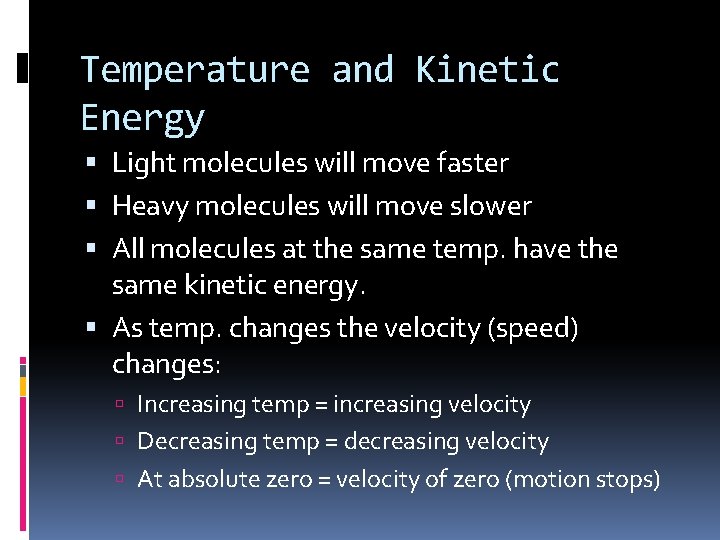
Temperature and Kinetic Energy Light molecules will move faster Heavy molecules will move slower All molecules at the same temp. have the same kinetic energy. As temp. changes the velocity (speed) changes: Increasing temp = increasing velocity Decreasing temp = decreasing velocity At absolute zero = velocity of zero (motion stops)

Gas laws: Avogadro’s hypothesis is Equal volumes of gases at the same temperature and pressure contain equal numbers of molecules In mathematical terms, the ratio of gas volume to moles is constant, if pressure and temperature do not change 20

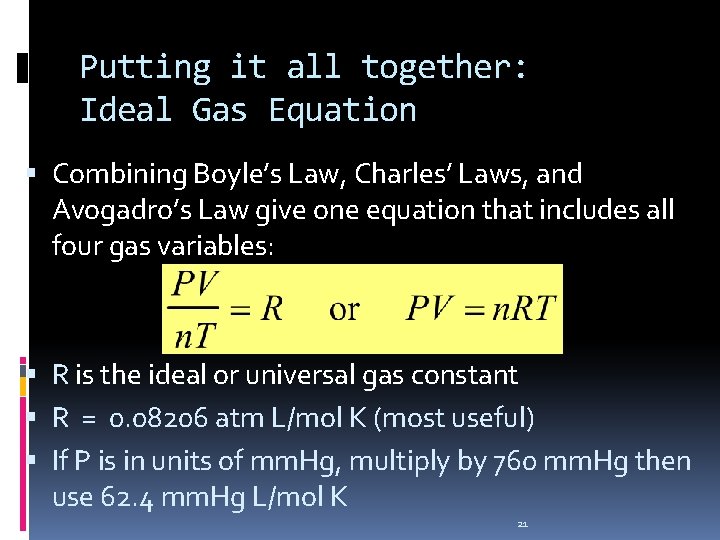
Putting it all together: Ideal Gas Equation Combining Boyle’s Law, Charles’ Laws, and Avogadro’s Law give one equation that includes all four gas variables: R is the ideal or universal gas constant R = 0. 08206 atm L/mol K (most useful) If P is in units of mm. Hg, multiply by 760 mm. Hg then use 62. 4 mm. Hg L/mol K 21

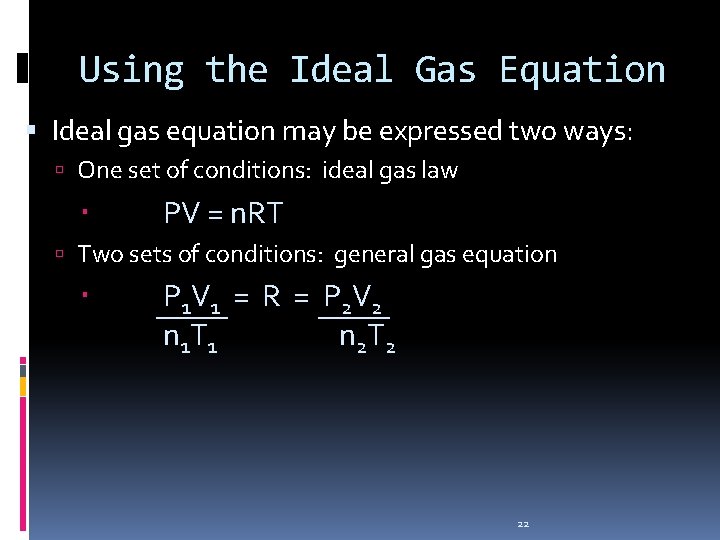
Using the Ideal Gas Equation Ideal gas equation may be expressed two ways: One set of conditions: ideal gas law PV = n. RT Two sets of conditions: general gas equation P 1 V 1 = R = P 2 V 2 n 1 T 1 n 2 T 2 22

Examples What is the volume occupied by 20. 2 g NH 3 gas at – 25 °C and 752 mm Hg? How many moles of He gas are in a 5. 00 L tank at 10. 5 atm pressure and 30. 0 °C? A 1. 00 m. L sample of N 2 gas at 36. 2 °C and 2. 14 atm is heated to 37. 8 °C while the pressure is changed to 1. 02 atm. What volume does the gas occupy at this temperature and pressure? 23

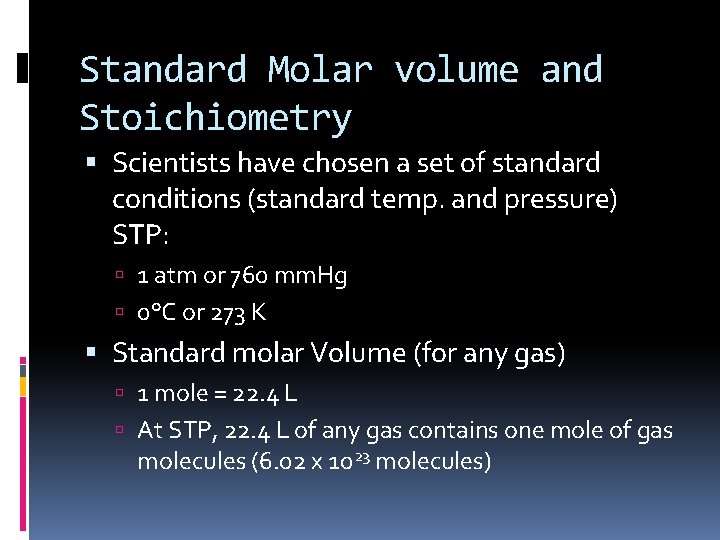
Standard Molar volume and Stoichiometry Scientists have chosen a set of standard conditions (standard temp. and pressure) STP: 1 atm or 760 mm. Hg 0°C or 273 K Standard molar Volume (for any gas) 1 mole = 22. 4 L At STP, 22. 4 L of any gas contains one mole of gas molecules (6. 02 x 1023 molecules)

Let’s Practice… Convert. 5 moles of gas into L Convert 12 L into moles

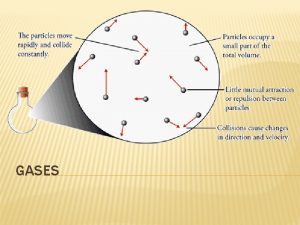
A Model for Gas Behavior The gas laws describe what gases do, but they do not explain why. The Kinetic Molecular Theory of Gases is the model that explains gas behavior. KMT was developed by Maxwell and Boltzmann in the mid -1800 s KMT is based on the concept of an ideal or perfect gas 26

Ideal gas Composed of tiny particles in constant, random, straight-line motion Gas molecules are point masses, so gas volume is just the empty space between the molecules Molecules collide with each other and with the walls of their container The molecules are completely independent of each other, with no attractive or repulsive forces between them. Individual molecules may gain or lose energy during collisions, but the total energy of the gas sample depends only on the absolute temperature. 27
 Four properties of gas
Four properties of gas Properties of gas
Properties of gas What are the different properties of gas?
What are the different properties of gas? Physical properties of gas
Physical properties of gas Physical properties and chemical properties
Physical properties and chemical properties Physical characteristics of gases
Physical characteristics of gases Physical characteristics of gases
Physical characteristics of gases Physical characteristics of gases
Physical characteristics of gases Solids liquids and gases section 2 properties of fluids
Solids liquids and gases section 2 properties of fluids Properties of solid
Properties of solid What are the general properties of gases
What are the general properties of gases 5 properties of gases
5 properties of gases Properties of gases
Properties of gases Characteristic of noble gas
Characteristic of noble gas Properties of gases
Properties of gases What is noble gas
What is noble gas Matter and its composition
Matter and its composition Properties of gasses
Properties of gasses List 2 of the important properties of gases
List 2 of the important properties of gases Pseudo reduced specific volume
Pseudo reduced specific volume Imaginary gas
Imaginary gas Differences between ideal gas and real gas
Differences between ideal gas and real gas Sutherland's law
Sutherland's law Conclusion on bhopal gas tragedy
Conclusion on bhopal gas tragedy Gas leaked in bhopal gas tragedy
Gas leaked in bhopal gas tragedy Gas reale e gas ideale
Gas reale e gas ideale Flue gas desulfurisation gas filter
Flue gas desulfurisation gas filter Poisonous gas leaked in bhopal gas tragedy
Poisonous gas leaked in bhopal gas tragedy