Intro to Gases Kinetic Molecular Theory of Gases






















- Slides: 44

Intro to Gases

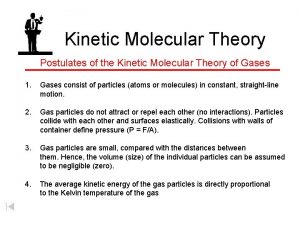
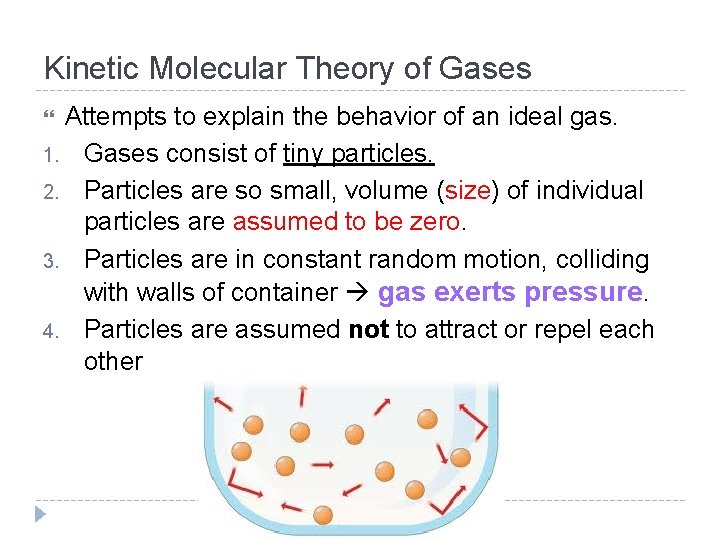
Kinetic Molecular Theory of Gases Attempts to explain the behavior of an ideal gas. 1. Gases consist of tiny particles. 2. Particles are so small, volume (size) of individual particles are assumed to be zero. 3. Particles are in constant random motion, colliding with walls of container gas exerts pressure. 4. Particles are assumed not to attract or repel each other

Kinetic Molecular Theory of Gases cont 5. Average kinetic energy of gas particle is directly proportional to Kelvin temperature of the gas.

How Temperature affects K. E. of a gas The _____ higher the temperature the _____ faster the particles Direct move, so the _____ more K. E. the particles have! (____ Relationship: As Temperature increases, K. E. increases. ) • At 0˚K, (_______ absolute zero ______), kinetic energy is also ____. zero • Doubling the Kelvin temperature would ______ double the K. E. (Twice as hot means ____ the ______ temperature. ) twice Kelvin

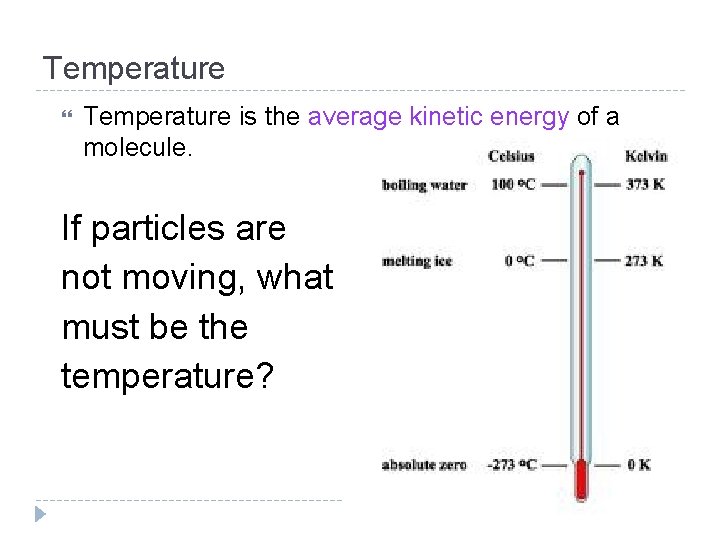
Temperature is the average kinetic energy of a molecule. If particles are not moving, what must be the temperature?

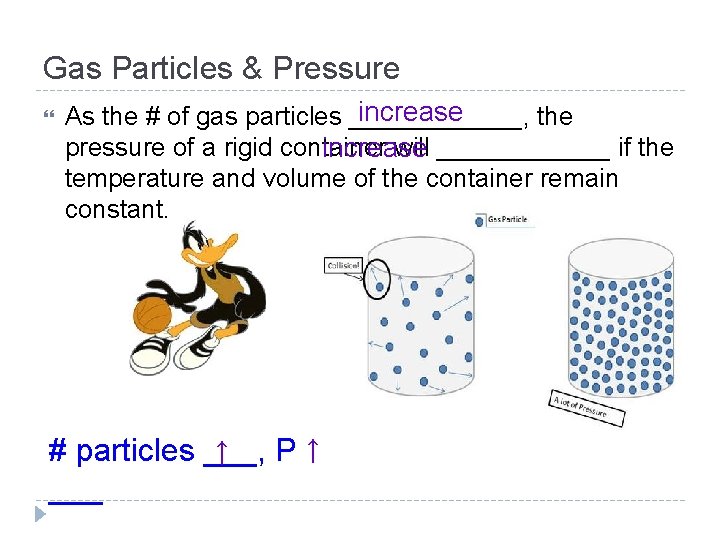
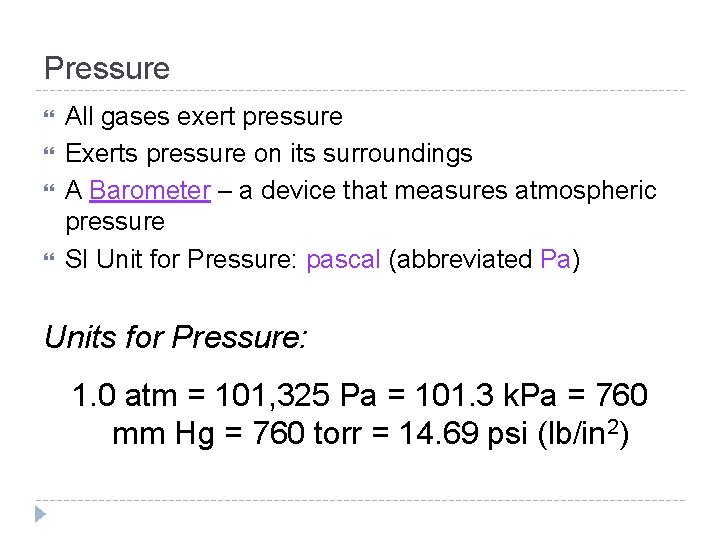
Gas Particles & Pressure increase As the # of gas particles ______, the pressure of a rigid container will ______ if the increase temperature and volume of the container remain constant. ↑ # particles ___, P↑ ___

Pressure All gases exert pressure Exerts pressure on its surroundings A Barometer – a device that measures atmospheric pressure SI Unit for Pressure: pascal (abbreviated Pa) Units for Pressure: 1. 0 atm = 101, 325 Pa = 101. 3 k. Pa = 760 mm Hg = 760 torr = 14. 69 psi (lb/in 2)

Gas Particles & Pressure

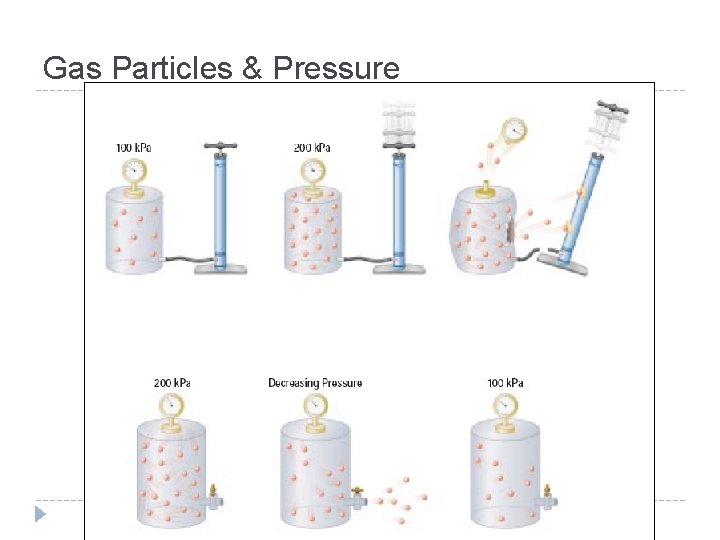
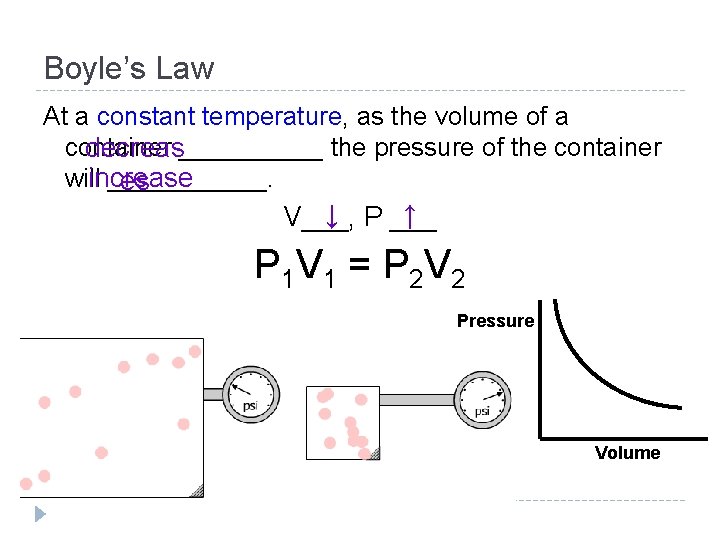
Boyle’s Law At a constant temperature, as the volume of a container decreas_____ the pressure of the container willincrease ______. es ↓ P ___ ↑ V___, Pressure Volume

Gases Particles & Volume What happens when we blow air into a balloon? As the # of gas particles _______, the volume increase of a flexible container willincrease ______ (if the temperature and pressure of the container remain constant) ↑ # particles ___, V ↑___

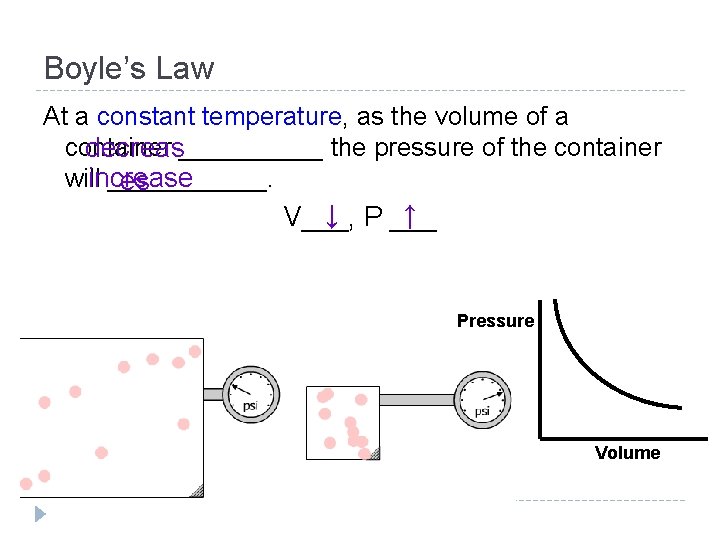
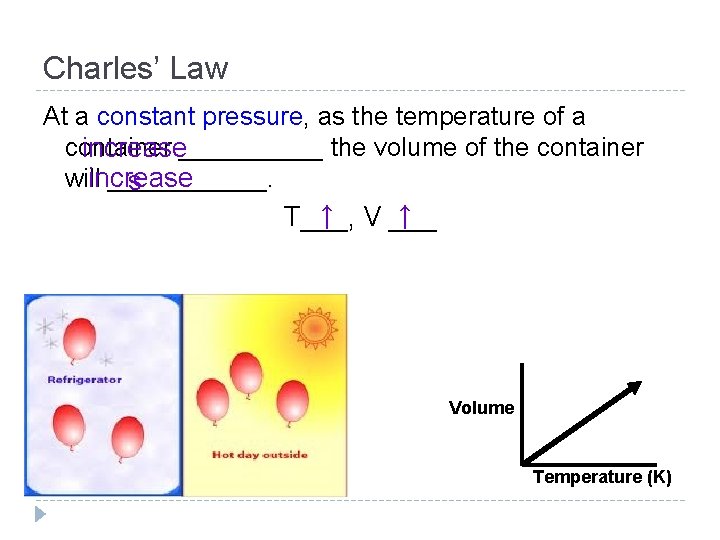
Charles’ Law At a constant pressure, as the temperature of a container increase_____ the volume of the container willincrease ______. s ↑ V ___ ↑ T___, Volume Temperature (K)

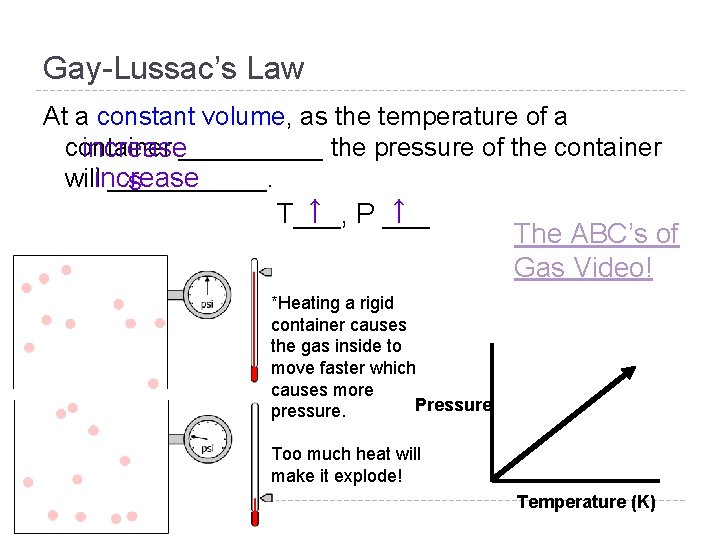
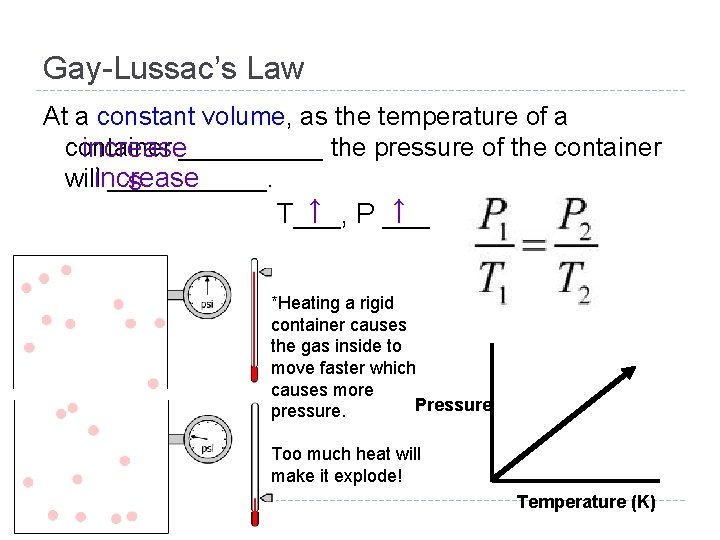
Gay-Lussac’s Law At a constant volume, as the temperature of a container increase_____ the pressure of the container willincrease ______. s ↑ P ___ ↑ T___, The ABC’s of Gas Video! *Heating a rigid container causes the gas inside to move faster which causes more Pressure pressure. Too much heat will make it explode! Temperature (K)

Exit Slip 1. If you increase the temperature, what happens to the speed of gas particles? 2. Explain what would happen to the size of a balloon on a hot day.

Gas Laws

Temperature Unit When doing calculations, we want our temperature in: KELVIN K = ˚C + 273 ˚C = K - 273 I am LORD KELVIN

Standard Temperature and Pressure (STP) Often the volume of a gas is needed at “standard conditions. ” For scientists, this means “STP”. 273 is ______K, and standard Standard temperature 1 pressure is equal to ___ atmosphere (atm)

Charles’ Law At a constant pressure, as the temperature of a container increase_____ the volume of the container willincrease ______. s ↑ V ___ ↑ T___, Volume Temperature (K)

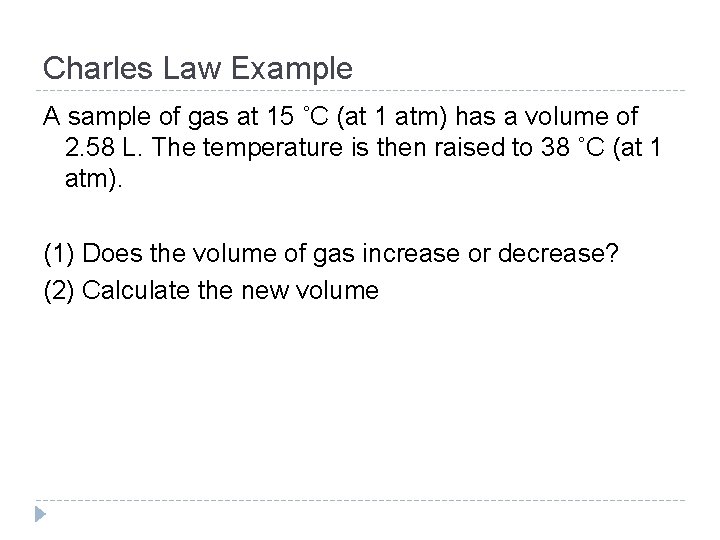
Charles Law Example A sample of gas at 15 ˚C (at 1 atm) has a volume of 2. 58 L. The temperature is then raised to 38 ˚C (at 1 atm). (1) Does the volume of gas increase or decrease? (2) Calculate the new volume

Gay-Lussac’s Law At a constant volume, as the temperature of a container increase_____ the pressure of the container willincrease ______. s ↑ P ___ ↑ T___, *Heating a rigid container causes the gas inside to move faster which causes more Pressure pressure. Too much heat will make it explode! Temperature (K)

Boyle’s Law At a constant temperature, as the volume of a container decreas_____ the pressure of the container willincrease ______. es ↓ P ___ ↑ V___, P 1 V 1 = P 2 V 2 Pressure Volume

1. Argon gas has a pressure of 2 atm at 35 ˚C (at constant volume), but the temperature was decreased to 20 ˚C. What is the new pressure? 2. A 2. 0 L sample of a gaseous compound is at a pressure of 1. 0 atm. If the volume is changed to 0. 75 L at a constant temperature, what is the new pressure?

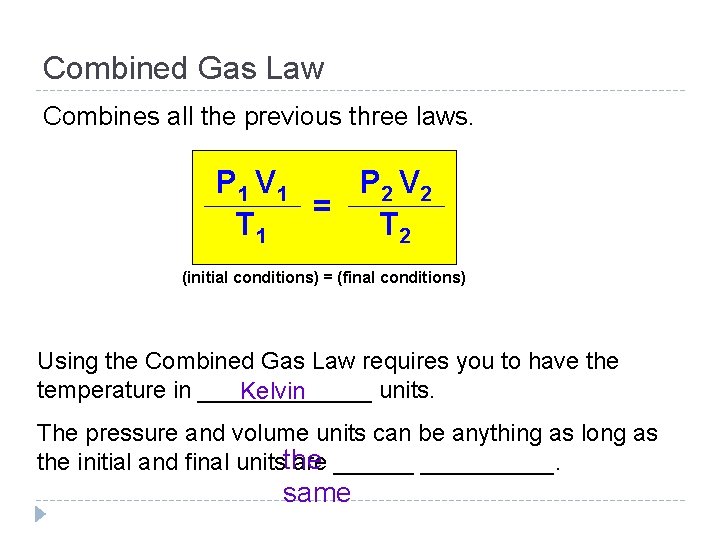
Combined Gas Law Combines all the previous three laws. P 1 V 1 P 2 V 2 = T 1 T 2 (initial conditions) = (final conditions) Using the Combined Gas Law requires you to have the temperature in _______ units. Kelvin The pressure and volume units can be anything as long as the initial and final unitsthe are __________. same

Exit Slip What unit should our temperature for gas laws be in? Convert 50 ˚C to that unit!

Exit Slip A sample of a gaseous compound has a pressure of 240 torr at 40˚C (at constant volume), but the temperature was decreased to 28 ˚C. What is the new pressure?

Gas Law Practice Side 1: 1) a. V b. P c. T d. T e. V f. P 2) Speed Up! Increase 3) Decreases, Boyle’s 4) Decreases, Gay-Lusaac 5) 16 L 6) Increases, 2100 mm Hg 7) Increase, 4 atm 8) 8. 0 atm 9) 10) Box 1 11) a. 44. 1 psi b. 60. 0 k. Pa c. 300, 074 Pa Side 2: 1) 348. 5 K 2) 1. 41 atm, G-L 3) 569 m. L, Charles 4) 1520 mm Hg, B 5) 0. 52 atm, G-L

Ideal Gas Law

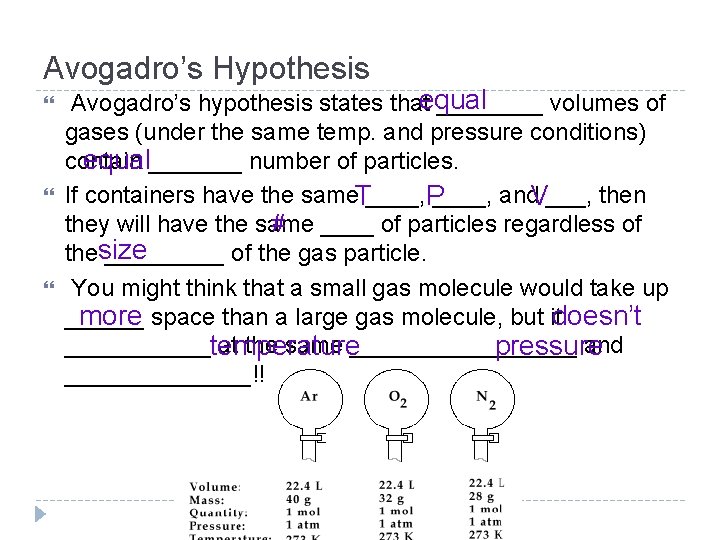
Avogadro’s Hypothesis Avogadro’s hypothesis states thatequal ____ volumes of gases (under the same temp. and pressure conditions) contain equal_______ number of particles. If containers have the same. T____, P ____, and. V___, then # ____ of particles regardless of they will have the same thesize _____ of the gas particle. You might think that a small gas molecule would take up ______ more space than a large gas molecule, but itdoesn’t ______temperature at the same _________ and pressure _______!!

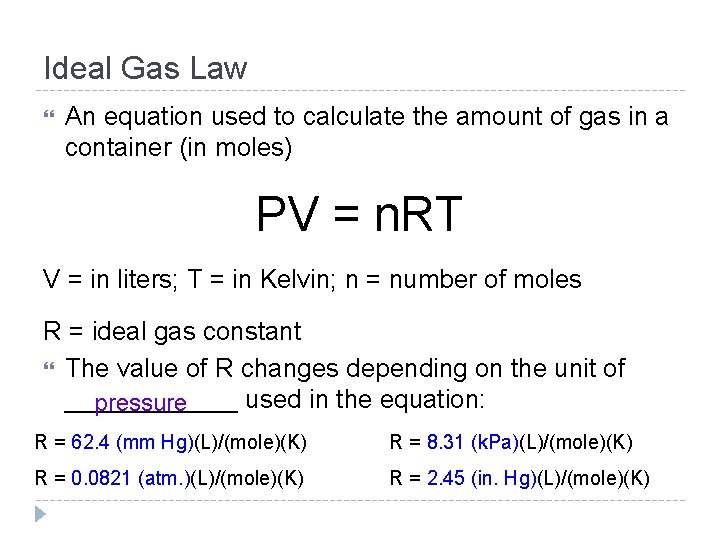
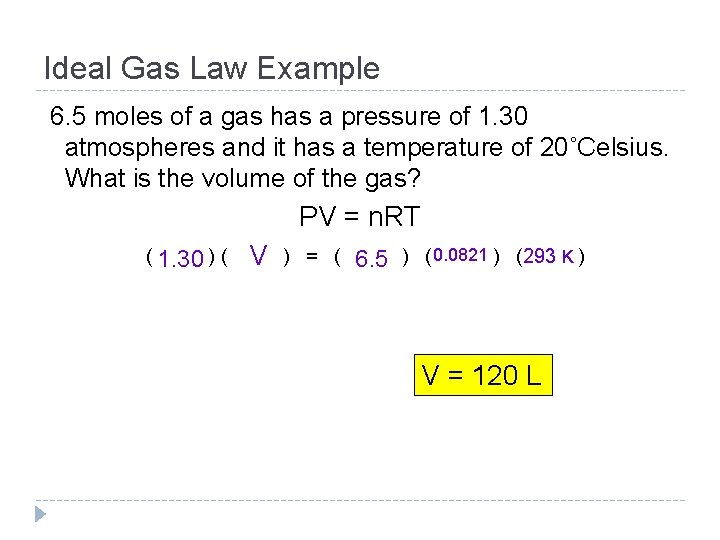
Ideal Gas Law An equation used to calculate the amount of gas in a container (in moles) PV = n. RT V = in liters; T = in Kelvin; n = number of moles R = ideal gas constant The value of R changes depending on the unit of ______ used in the equation: pressure R = 62. 4 (mm Hg)(L)/(mole)(K) R = 8. 31 (k. Pa)(L)/(mole)(K) R = 0. 0821 (atm. )(L)/(mole)(K) R = 2. 45 (in. Hg)(L)/(mole)(K)

Ideal Gas Law Example 6. 5 moles of a gas has a pressure of 1. 30 atmospheres and it has a temperature of 20˚Celsius. What is the volume of the gas? PV = n. RT ( 1. 30 ) ( V ) = ( 6. 5 ) ( 0. 0821 ) (293 K ) V = 120 L

Practice Ideal Gas Problem How many moles of gas are there in a 7. 3 liter balloon with a pressure of 1. 5 atm and temperature of 395 K?

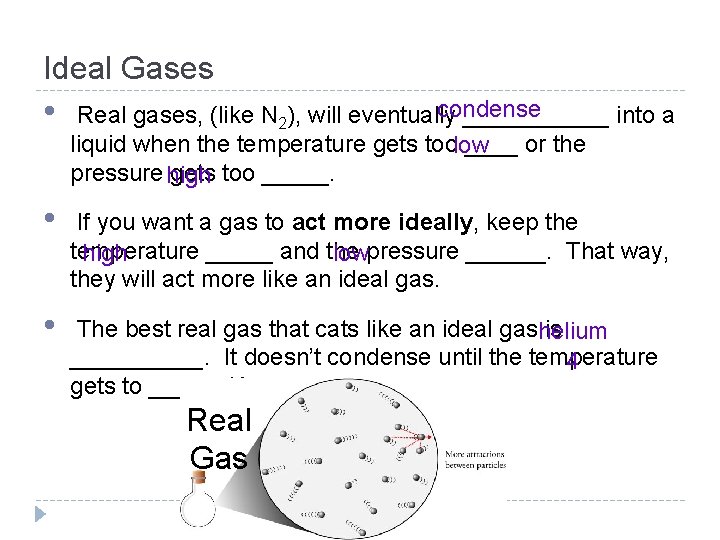
Ideal Gases • condense Real gases, (like N 2), will eventually ______ into a liquid when the temperature gets toolow ____ or the pressure high gets too _____. • If you want a gas to act more ideally, keep the temperature _____ and the high lowpressure ______. That way, they will act more like an ideal gas. • The best real gas that cats like an ideal gashelium is _____. It doesn’t condense until the temperature 4 gets to ______K. Real Gas

Exit Slip How big is a balloon if the balloon is filled with 2 moles of nitrogen gas and has a pressure of 0. 5 atm at a temperature of 350 K?

Pressure & Dalton’s Law of Partial Pressure

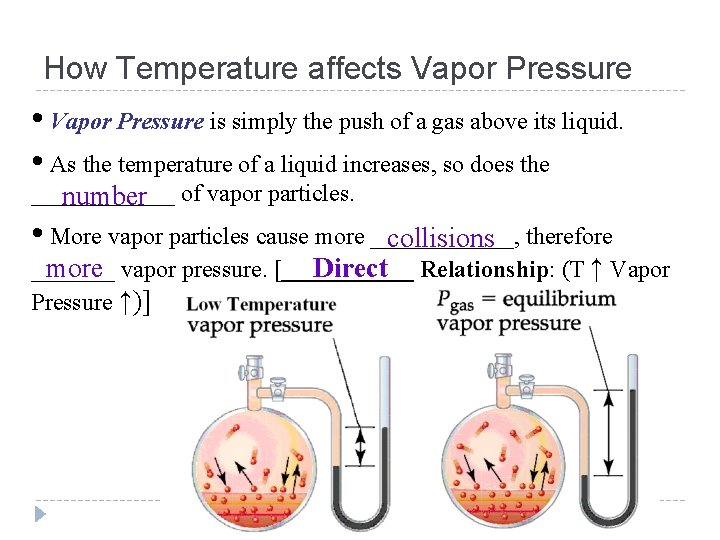
How Temperature affects Vapor Pressure • Vapor Pressure is simply the push of a gas above its liquid. • As the temperature of a liquid increases, so does the ______ number of vapor particles. • More vapor particles cause more ______, collisions therefore _______ more vapor pressure. [______ Direct Relationship: (T ↑ Vapor Pressure ↑)]

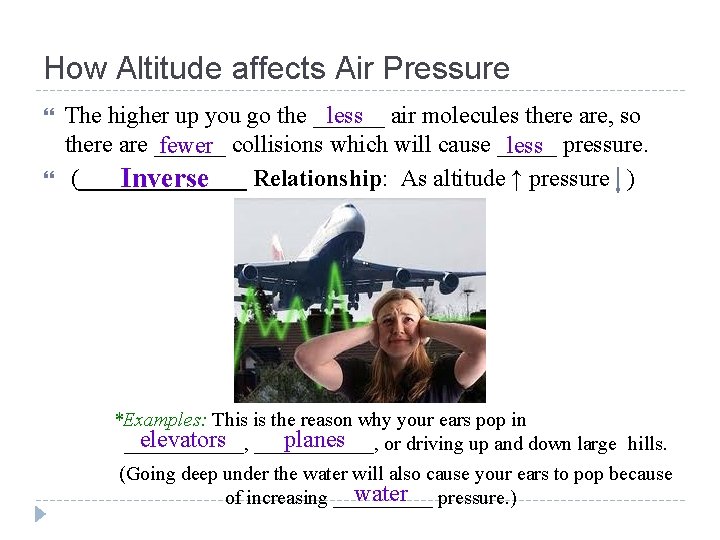
How Altitude affects Air Pressure less air molecules there are, so The higher up you go the ______ there are ______ fewer collisions which will cause _____ less pressure. (_______ Relationship: As altitude ↑ pressure ) Inverse *Examples: This is the reason why your ears pop in elevators ______, planes ______, or driving up and down large hills. (Going deep under the water will also cause your ears to pop because water pressure. ) of increasing _____

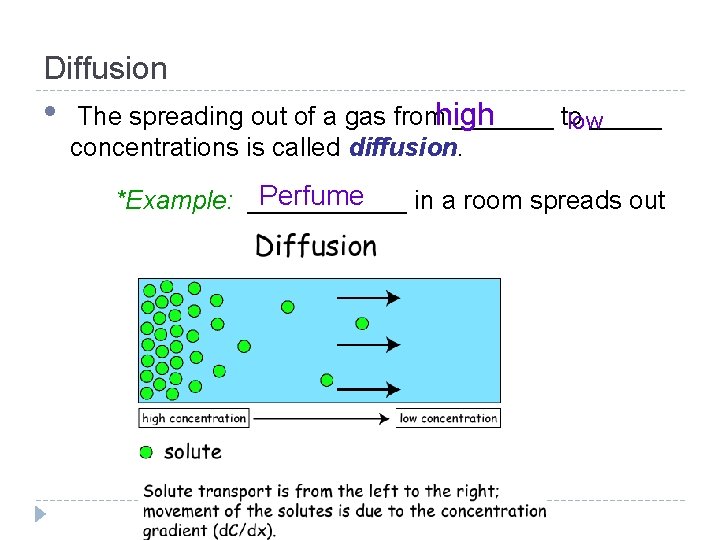
Diffusion • The spreading out of a gas fromhigh _______ to _____ low concentrations is called diffusion. Perfume *Example: ______ in a room spreads out

Dalton’s Law of Partial Pressure • The ______ sum of each individual gas pressure equals thetotal _______ gas pressure of the container. P(total)= P 1+P 2+P 3… For a mixture of ideal gases, it is the number of moles of particles that is important, not identity.

Partial Pressures Example A container has 1 mole of oxygen and 3 moles of helium in it. The total pressure of the container is 2. 4 atmospheres. What is the partial pressure of oxygen and helium?

Exit Slip A balloon has a mix of 3 moles of oxygen gas and 4 moles of helium gas. If the total pressure is 9. 0 atm, what is the partial pressure of each gas?

Few selected answers from last year

Gas Law Practice 3 (Check yo answers!) 5. Box 1 6. 569 m. L (remember temp should be in Kelvin!) 7. Box 2 8. 1520 mm Hg *can cross out one 9. 3 atm of the #10’s 10. 7. 76 psi 10. 200 K 11. 0. 524 atm 12. 253. 25 k. Pa 13. 4. 83 psi 14. 11. 4 K

Acc Gas Law Practice 3 (Check yo answers!) 4. Box 1 5. 569 m. L 6. Box 2 7. 1520 mm Hg 8. 3 atm 9. 7. 76 psi 10. 160. 4 K 11. 200 K 12. 29. 6 L 13. 25 k. Pa 14. 4. 83 psi 15. 11. 4 K

Gas Law Practice 4 3. 41. 0 gallons 4. 2 L (this one is tricky!!!) normally its 4 L but it’s a closed jar, so the jar can’t expand (if there is enough pressure the can would _____) 5. a) 0. 95 moles b) 77. 9 L c) 406 K

Answers to Gas Law Practice 5 2. Air P decreases (less air molecules) 3. Heating more gas particles more particles mean more pressure 4. PN 2 = 2. 96 atm PH 2 = 1. 97 atm Ptot = 4. 93 atm 5. PCO 2 = 0. 583 atm b. PH 2 = 0. 117 atm 7. PO 2 = 2. 4 atm PHe = 9. 3 atm Ptot = 11. 7 atm (the number of moles is 0. 49 mol O 2 and 1. 9 mol He it’s in a new container with a volume of 5. 0 L)
 Kinetic molecular theory of gases
Kinetic molecular theory of gases Kinetic molecular theory of solids
Kinetic molecular theory of solids The kinetic molecular theory
The kinetic molecular theory Kinetic molecular theory of liquids and solids
Kinetic molecular theory of liquids and solids Kinetic molecular theory of liquids
Kinetic molecular theory of liquids Postulates of kinetic molecular theory
Postulates of kinetic molecular theory Kinetic theory def
Kinetic theory def Kinetic molecular theory timeline
Kinetic molecular theory timeline Charles law in terms of kinetic molecular theory
Charles law in terms of kinetic molecular theory Kinetic theory of gases postulates
Kinetic theory of gases postulates Kenitic molecular theory
Kenitic molecular theory Postulates of kinetic theory of gases
Postulates of kinetic theory of gases Pv=1/3nmc^2
Pv=1/3nmc^2 Kinetic molecular theory
Kinetic molecular theory Tenets of kinetic molecular theory
Tenets of kinetic molecular theory Condensation particle theory
Condensation particle theory Kinetic theory of gases
Kinetic theory of gases Kinetic theory of gases
Kinetic theory of gases Write postulates of kinetic theory of gases
Write postulates of kinetic theory of gases Kinetic theory of gases
Kinetic theory of gases Molecular theory of gases and liquids
Molecular theory of gases and liquids Difference between vbt and mot
Difference between vbt and mot Vb theory vs mo theory
Vb theory vs mo theory Valence bond theory and molecular orbital theory
Valence bond theory and molecular orbital theory 11:55
11:55 Atomic orbitals of oxygen
Atomic orbitals of oxygen Covalent bond melting point
Covalent bond melting point Ionic covalent metallic
Ionic covalent metallic Zinc oxide + nitric acid → zinc nitrate + water
Zinc oxide + nitric acid → zinc nitrate + water Teoría cinético molecular de los gases
Teoría cinético molecular de los gases Pengantar teori permainan
Pengantar teori permainan The kinetic theory of matter states that
The kinetic theory of matter states that Kinetic theory of matter definition
Kinetic theory of matter definition Kinetic theory of matter
Kinetic theory of matter An explanation of how particles in matter behave
An explanation of how particles in matter behave Kinetic theory of solids
Kinetic theory of solids What is a kinetic theory of matter
What is a kinetic theory of matter Dicots
Dicots Site:slidetodoc.com
Site:slidetodoc.com Neutral theory of molecular evolution notes
Neutral theory of molecular evolution notes Atomic molecular theory
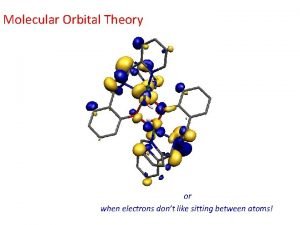
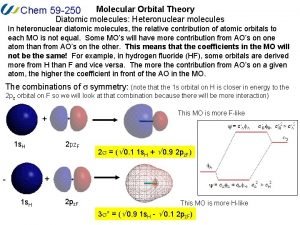
Atomic molecular theory Heteronuclear diatomic molecules molecular orbital diagram
Heteronuclear diatomic molecules molecular orbital diagram Vsepr theory molecular shapes
Vsepr theory molecular shapes Molecular orbital theory
Molecular orbital theory Frontier molecular orbital theory
Frontier molecular orbital theory