Atomic Molecular Theory of Matter The Atomic Molecular







- Slides: 16


Atomic - Molecular Theory of Matter The Atomic - Molecular Theory of Matter states that all matter is composed of small, fast moving particles called atoms. These atoms can join together to form molecules. This theory is really thousands of individual theories that provide evidence for the whole theory.

History of Atom All atoms share the same basic structure l During past 200 years, scientists have proposed different models l

Where did it all begin? The word “atom” comes from the Greek word “atomos” which means indivisible. The idea that all matter is made up of atoms was first proposed by the Greek philosopher Democritus in the 5 th century B. C.

Dalton’s Model Based on experiments, Dalton developed a theory of structure of matter l 4 main concepts: l All matter is composed of tiny, indivisible particles called atoms l Atoms of each element are exactly alike and have the same mass l An atom of one element cannot be changed into an atom of a different element. l Atoms of different elements can join to form compounds. l

Dalton’s Model = “eight ball” Dalton thought that atoms were like smooth, hard balls that could not be broken into smaller pieces.

Thomson’s Model End of 1800 s l Thomson discovered that atoms were not simple, solid spheres l Atoms contained subatomic particles l l Very small, negatively charged l Called them electrons

Thomson’s Model l Also knew that atoms were electrically neutral l Must contain enough positive charge to balance negative charge of electrons l Thompson proposed a model where electrons were stuck into a positively charged sphere l Like chocolate chips in cookie dough

Thomson’s Model = chocolate chip cookie

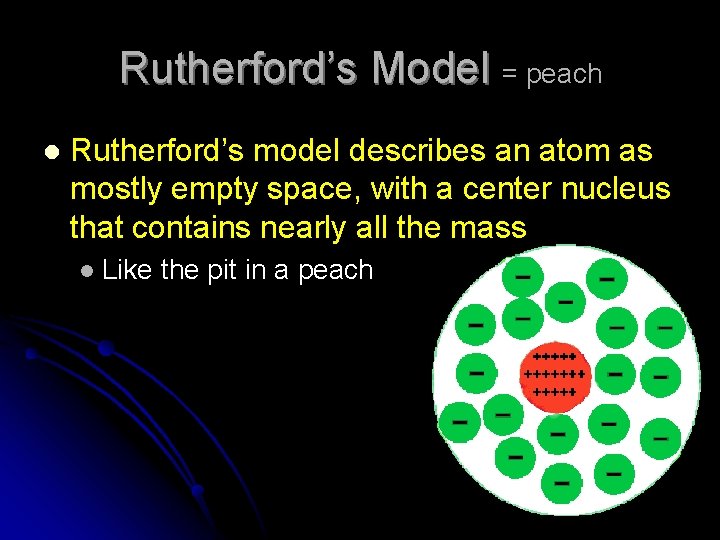
Rutherford’s Model By early 1900 s, scientists knew that positive charge of atom comes from subatomic particles called protons l 1911—Rutherford begins to test theory l His experiments led him to believe that protons are concentrated in a small area at center of atom l l Called this area the nucleus

Rutherford’s Model = peach l Rutherford’s model describes an atom as mostly empty space, with a center nucleus that contains nearly all the mass l Like the pit in a peach

Bohr’s Model Modified Rutherford’s model in 1913 l Proposed that each electron has a certain amount of energy l l Helped electron move around nucleus Electrons move around nucleus in region called energy levels l Energy levels surround nucleus in rings, like layers of onion l

Bohr’s Model = planets l Has been called planetary model l Energy levels occupied by electrons are like orbits of planets at different distances from the sun (nucleus)

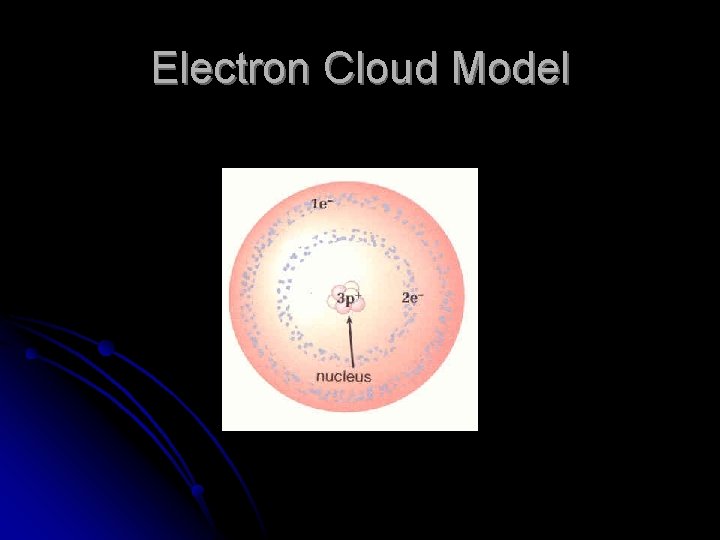
Electron Cloud Model accepted today l Electrons dart around in an energy level l Rapid, random motion creates a “cloud” of negative charge around nucleus l Electron cloud gives atom its size and shape l

Electron Cloud Model

Modern Atomic Model In 1932, Chadwick discovered another particle in the nucleus of an atom. This new particle is called a neutron. l Neutrons have no electrical charge. l According to this theory, “At the center of the atom is a tiny, massive nucleus containing protons and neutrons. Surrounding the nucleus is a cloudlike region of moving electrons. ” l