Gases and Liquids Fluids Kinetic Molecular Theory Gases




















- Slides: 35

Gases and Liquids Fluids

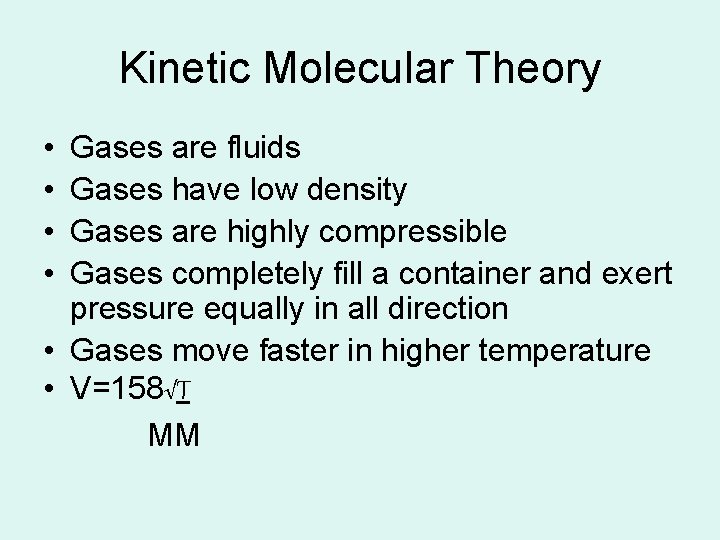
Kinetic Molecular Theory • • Gases are fluids Gases have low density Gases are highly compressible Gases completely fill a container and exert pressure equally in all direction • Gases move faster in higher temperature • V=158√T MM

Atmosphere is a sea of gases • Atmosphere

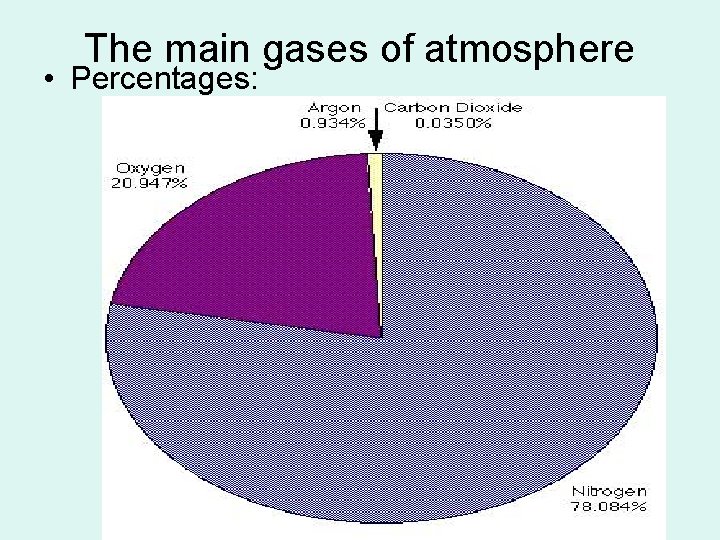
The main gases of atmosphere • Percentages:

Atmospheric pressure • Pressure= force on a surface divided by the area of that surface. Its unit in SI Pascal. • Pascal = 1 Newton of force meter squared

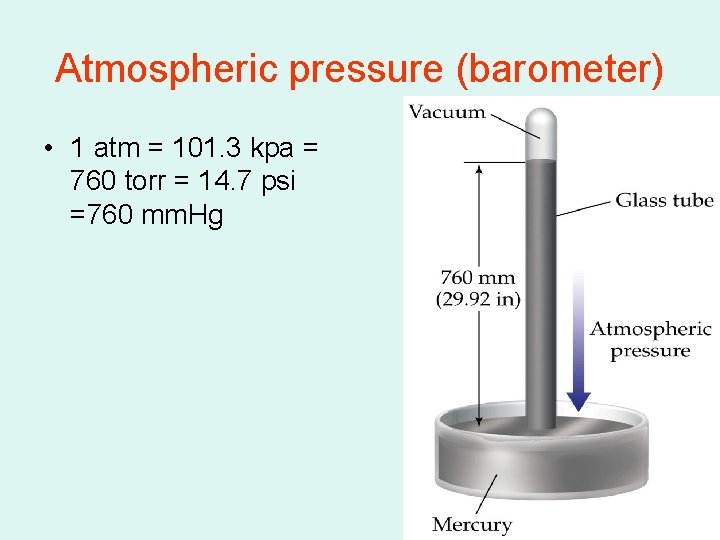
Atmospheric pressure (barometer) • 1 atm = 101. 3 kpa = 760 torr = 14. 7 psi =760 mm. Hg

Standard temperature and pressure • Standard temperature and pressure STP • STP = 0°C and 1 atm • At sea level the atmospheric pressure is equal to 1 atm. • Atmospheric pressure is lower in a higher altitude and higher in the valley

Pressure conversion • Convert 22 atm into Psi • 22 atm X 14. 7 psi = 323. 4 Psi 1 atm Convert 73 k. Pa into torr 73 k. Pa X 760 torr = 547. 7 torr 101, 3 k. Pa

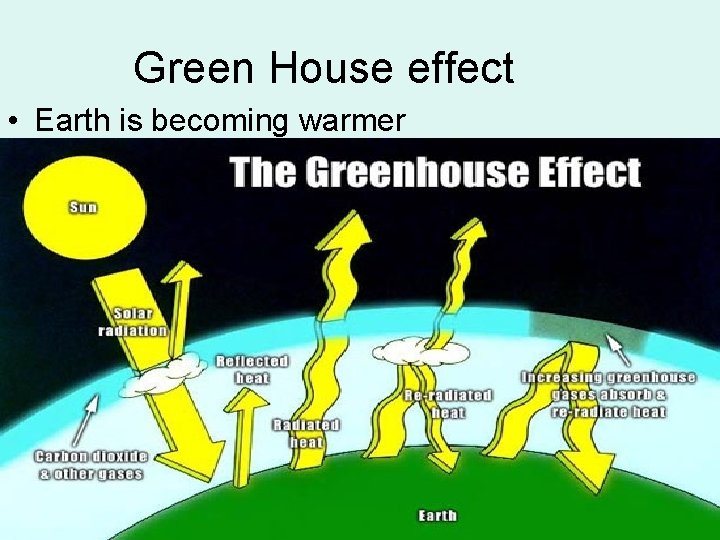
Green House effect • Earth is becoming warmer

Ozone depletion and free radicals • Free radicals such as Chlorine atom

Gas Laws • 1) Boyles law: The volume of a gas at constant temperature is inversely proportional to the pressure. P 1 V 1 =P 2 V 2

Problem • A sample of a gas has a volume of 100 m. L when its pressure is 0. 947 atm. What will the volume of the gas be at a pressure of 0. 987 atm, if the temperature remains constant? P 1 V 1 =P 2 V 2 • 95. 9 m. L

Dalton’s Law (partial pressure) • The total pressure is equal to the sum of partial pressure. Ptot= P 1+ P 2 +…

Problem • At a certain temperature, the vapor pressure of water is 20 mm. Hg. A gas is collected over water at the same temperature at a total pressure of 720 mm Hg. The partial pressure of the gas in mm. Hg is. . • 700 mm. Hg or torr

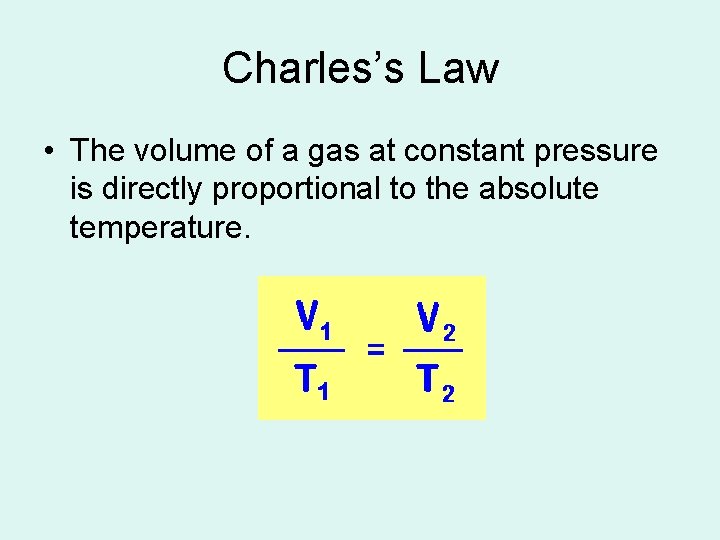
Charles’s Law • The volume of a gas at constant pressure is directly proportional to the absolute temperature.

Charles’s Law • Hot air balloon

Problem • A gas has a volume of 18 centimeter cube at 25 degree Celsius. If the temperature is increased to 35 C at constant pressure, the new volume will be. . • (Hint) T→ Kelvin = 18. 6 cm 3

Avogadro’s Law • Gases with equal volumes under the same conditions have an equal number of molecules. • I mole of any gas at STP occupies 22. 4 liter of volume. • 22. 4 liter of any gas at STP has the same number of molecules or atoms

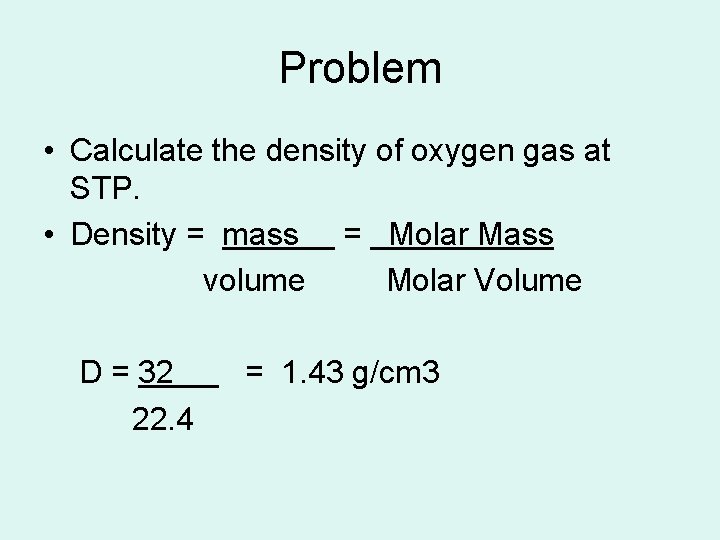
Problem • Calculate the density of oxygen gas at STP. • Density = mass = Molar Mass volume Molar Volume D = 32 22. 4 = 1. 43 g/cm 3

Lussac’s law of combining volume • Gases at constant temperature and pressure form compounds with each other in definite proportions

Lussac’s Law continues

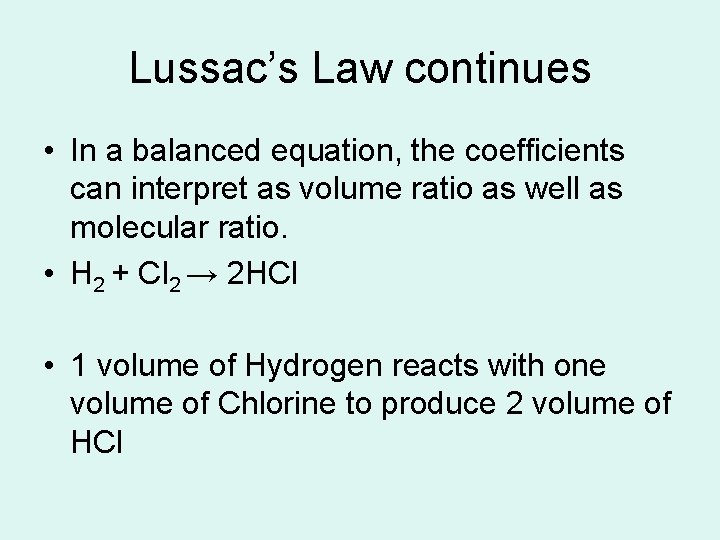
Lussac’s Law continues • In a balanced equation, the coefficients can interpret as volume ratio as well as molecular ratio. • H 2 + Cl 2 → 2 HCl • 1 volume of Hydrogen reacts with one volume of Chlorine to produce 2 volume of HCl

Problem • According to the Lussac’s law during the decomposition of the Ammonia (NH 3) to nitrogen gas and Hydrogen gas, what is the volume ratio of the Nitrogen gas to the hydrogen gas in that order? • 1 to 3

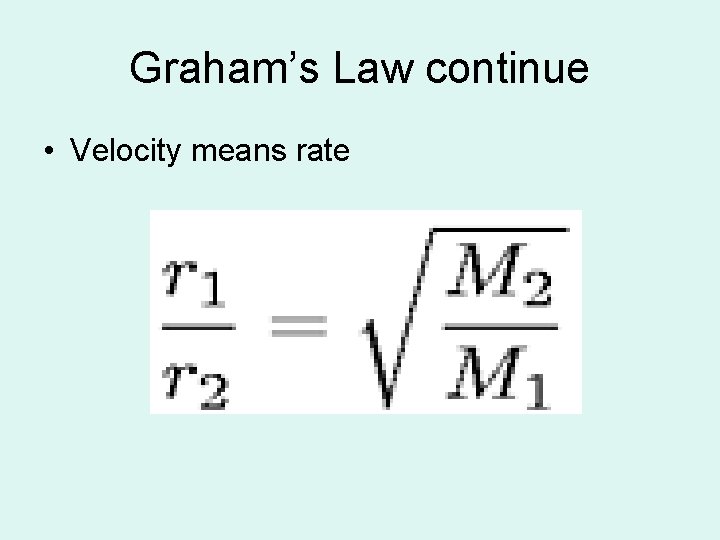
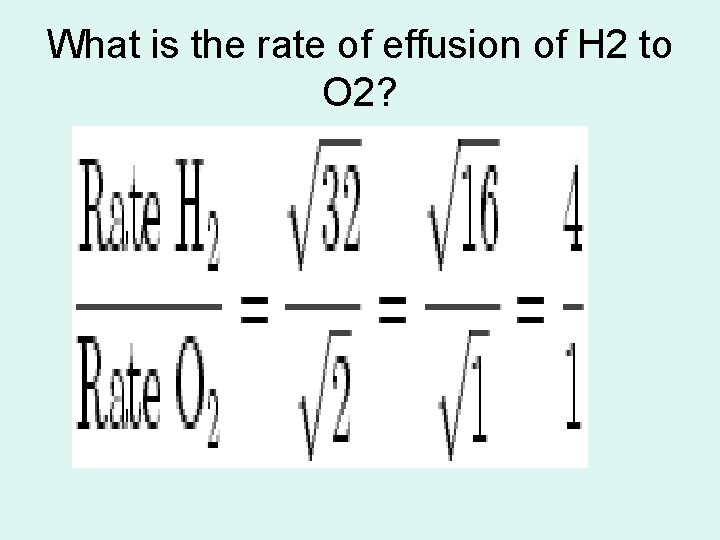
Graham’s Law( the law of diffusion) • At STP the rate of effusion (diffusion of gasses is inversely proportional to the square root of their molar masses.

Graham’s Law continue • Velocity means rate

What is the rate of effusion of H 2 to O 2?

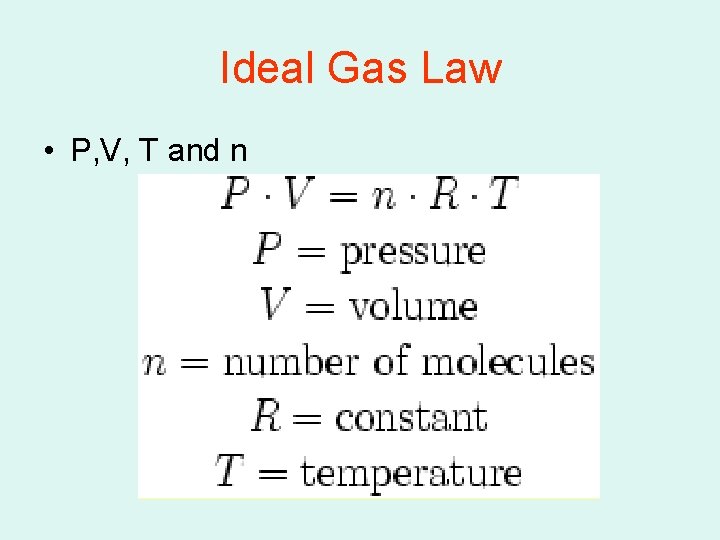
Ideal Gas Law • P, V, T and n

Ideal Gas law continues • If the Pressure is kpa R= 8. 314 L. kpa mol. K • If the Pressure is atm R= 0. 0821 L. atm mol. K

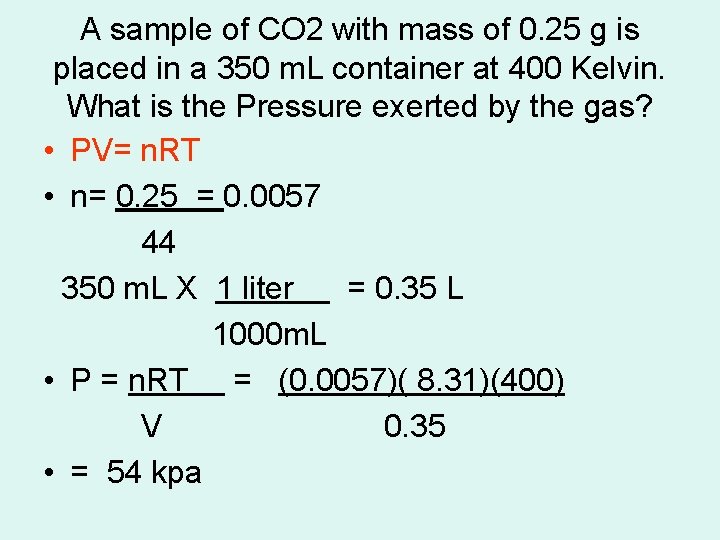
A sample of CO 2 with mass of 0. 25 g is placed in a 350 m. L container at 400 Kelvin. What is the Pressure exerted by the gas? • PV= n. RT • n= 0. 25 = 0. 0057 44 350 m. L X 1 liter = 0. 35 L 1000 m. L • P = n. RT = (0. 0057)( 8. 31)(400) V 0. 35 • = 54 kpa

Combine law

A sample of oxygen gas has a volume of 7. 84 cm 3 at a pressure of 71. 8 k. Pa and a temperature of 25 degrees Celsius. What will be the volume of the pressure changes to 101 k. Pa and the temperature goes down to 0 degrees Celsius? • The formula for combined law is • P 1 V 1 = P 2 V 2 T 1 T 2

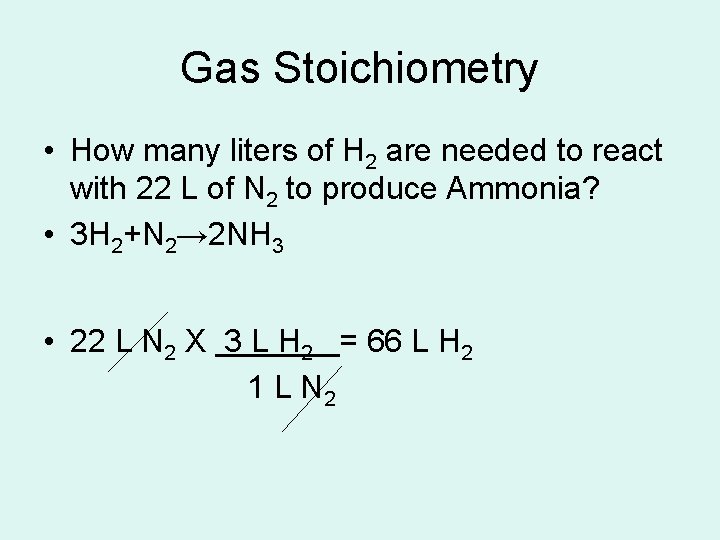
Gas Stoichiometry • How many liters of H 2 are needed to react with 22 L of N 2 to produce Ammonia? • 3 H 2+N 2→ 2 NH 3 • 22 L N 2 X 3 L H 2 = 66 L H 2 1 L N 2

Forces of attraction • Vapor pressure increases with temperature • H 2 O (l) ↔ H 2 O (g) water changes into gas in an open container (evaporation). The opposite of evaporation is condensation. • In a closed container the pressure due to water vapor reaches maximum value which is called vapor pressure.

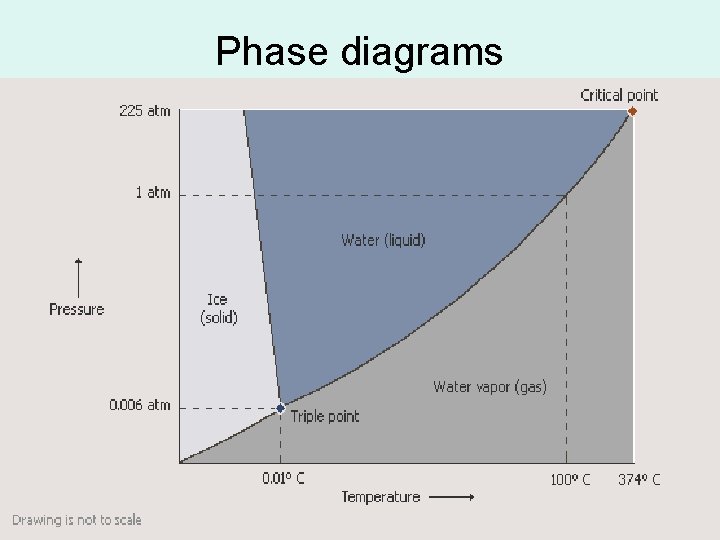
Phase diagrams

Phase diagrams • Critical point: At 374°C and 22. 1 MPa (218 atm) liquid water and vapor have the same density. • Normal boiling point for water is 100°C and 101 k. Pa. • Triple point of water is the point that three phases of water exist at equilibrium. The triple point of water is 0. 01°C and 0. 612 k. Pa. The sublimation region of the graph always lies below the triple point.
 Kinetic molecular theory of solids
Kinetic molecular theory of solids Kinetic molecular theory of liquids and solids
Kinetic molecular theory of liquids and solids Solids liquids and gases section 2 properties of fluids
Solids liquids and gases section 2 properties of fluids Molecular theory of gases and liquids
Molecular theory of gases and liquids Kinetic molecular theory of liquids
Kinetic molecular theory of liquids Kinetic molecular theory of gases
Kinetic molecular theory of gases Kinetic molecular theory volume
Kinetic molecular theory volume Kinetic molecular theory
Kinetic molecular theory Kinetic molecular theory def
Kinetic molecular theory def Theory vs hypothesis
Theory vs hypothesis Charles law in terms of kinetic molecular theory
Charles law in terms of kinetic molecular theory Kinetic molecular theory postulates
Kinetic molecular theory postulates Ideal gas law examples
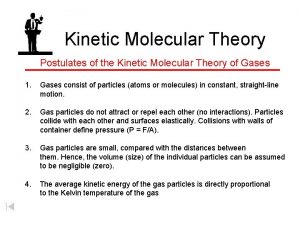
Ideal gas law examples Write the postulates of kinetic theory of gases
Write the postulates of kinetic theory of gases Equation of state of real gas
Equation of state of real gas Kinetic molecular theory
Kinetic molecular theory Tenets of kinetic molecular theory
Tenets of kinetic molecular theory Freezing particle theory
Freezing particle theory Kinetic theory of gases
Kinetic theory of gases Kinetic theory of gases
Kinetic theory of gases Write the postulates of kinetic theory of gases
Write the postulates of kinetic theory of gases Kinetic theory of gases
Kinetic theory of gases Expansion of solids liquids and gases examples
Expansion of solids liquids and gases examples Solid liquid gas venn diagram
Solid liquid gas venn diagram Mass of solid liquid and gas
Mass of solid liquid and gas Examples of solids liquids and gases pictures
Examples of solids liquids and gases pictures The science duo physical and chemical changes
The science duo physical and chemical changes Chapter 14 solids liquids and gases worksheet answers
Chapter 14 solids liquids and gases worksheet answers Particle movement in solids liquids and gases
Particle movement in solids liquids and gases How does sound travel through solids liquids and gases
How does sound travel through solids liquids and gases Properties of solids liquids and gases
Properties of solids liquids and gases Motion of particles in solids, liquids and gases
Motion of particles in solids, liquids and gases Why is gas easier to compress than liquid and solid
Why is gas easier to compress than liquid and solid Mot and vbt difference
Mot and vbt difference Valence bond theory and molecular orbital theory
Valence bond theory and molecular orbital theory Valence bond theory and molecular orbital theory
Valence bond theory and molecular orbital theory