GAS LAWS The Nature of Gases q Gases









- Slides: 21

GAS LAWS

The Nature of Gases q. Gases expand to fill their containers q. Gases are fluid – they flow q. Gases have low density q 1/1000 the density of the equivalent liquid or solid q. Gases are compressible q. Gases effuse and diffuse

Kinetic Theory • Gases are composed of small, separate particles called molecules • Move in constant motion • All collisions between particles are perfectly elastic • The molecules of a gas display no attraction or repulsion • The average kinetic energy of the molecules is directly proportional to Kelvin temperature of the gas

Ideal Gases Ideal gases are imaginary gases that perfectly fit all of the assumptions of the kinetic molecular theory. Ø Gases consist of tiny particles that are far apart relative to their size. Ø Collisions between gas particles and between particles and the walls of the container are elastic collisions Ø No kinetic energy is lost in elastic collisions

Ideal Gases (continued) q Gas particles are in constant, rapid motion. They therefore possess kinetic energy, the energy of motion q There are no forces of attraction between gas particles q The average kinetic energy of gas particles depends on temperature, not on the identity of the particle.

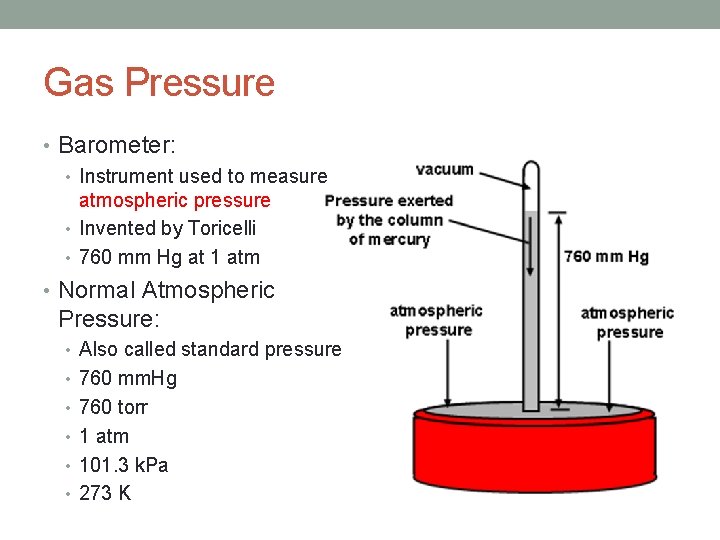
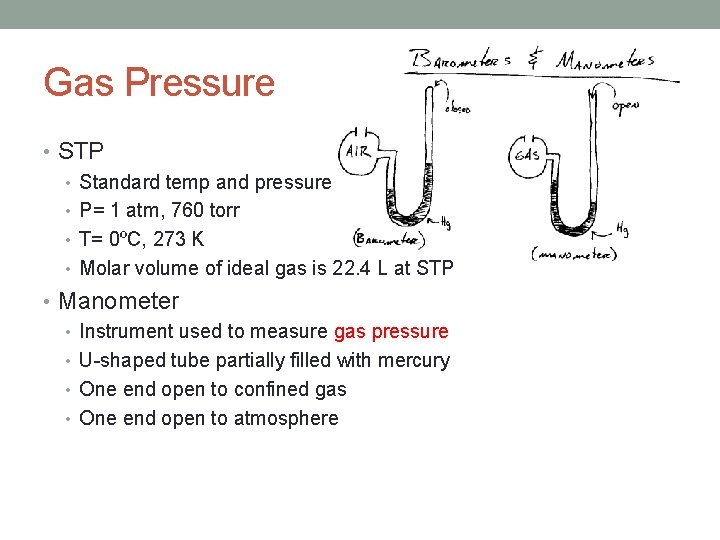
Gas Pressure • Pressure= Force/Area • Atmospheric pressure: • The pressure the earth’s atmosphere exerts due to its weight • Barometer: • Instrument used to measure atmospheric pressure • Invented by Toricelli • Baro= weight • Meter= measure • Normal Atmospheric Pressure: • Also called standard pressure • 1 atm = 760 mm Hg = 760 torr • 1 atm= 101. 3 k. Pa

Gas Pressure • Barometer: • Instrument used to measure atmospheric pressure • Invented by Toricelli • 760 mm Hg at 1 atm • Normal Atmospheric Pressure: • Also called standard pressure • 760 mm. Hg • 760 torr • 1 atm • 101. 3 k. Pa • 273 K

Gas Pressure • STP • Standard temp and pressure • P= 1 atm, 760 torr • T= 0ºC, 273 K • Molar volume of ideal gas is 22. 4 L at STP • Manometer • Instrument used to measure gas pressure • U-shaped tube partially filled with mercury • One end open to confined gas • One end open to atmosphere

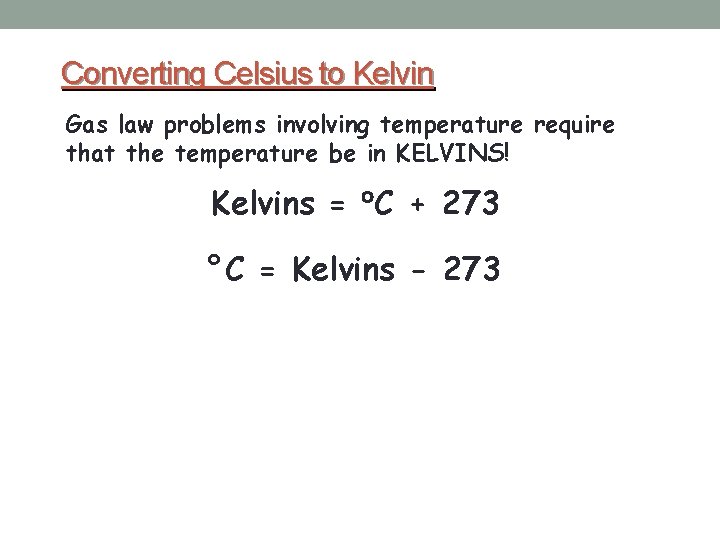
Converting Celsius to Kelvin Gas law problems involving temperature require that the temperature be in KELVINS! Kelvins = C + 273 °C = Kelvins - 273

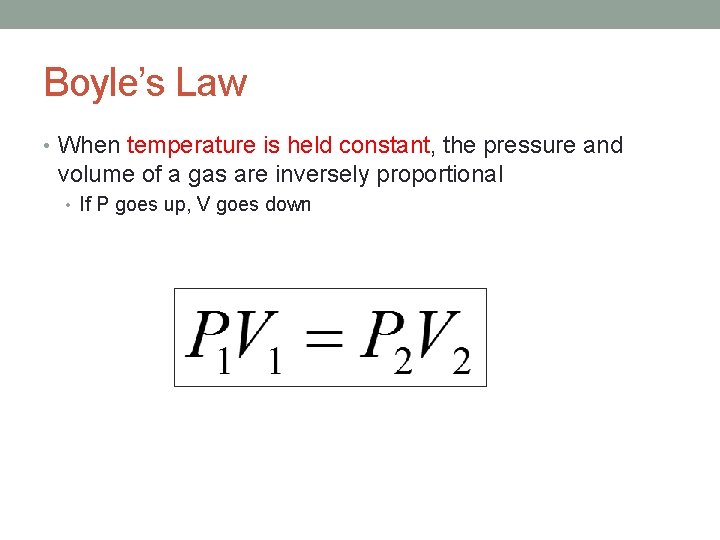
Boyle’s Law • When temperature is held constant, the pressure and volume of a gas are inversely proportional • If P goes up, V goes down

Charles’s Law • When pressure is held constant, the volume and temperature of a gas are directly proportional • If V goes up, T goes up

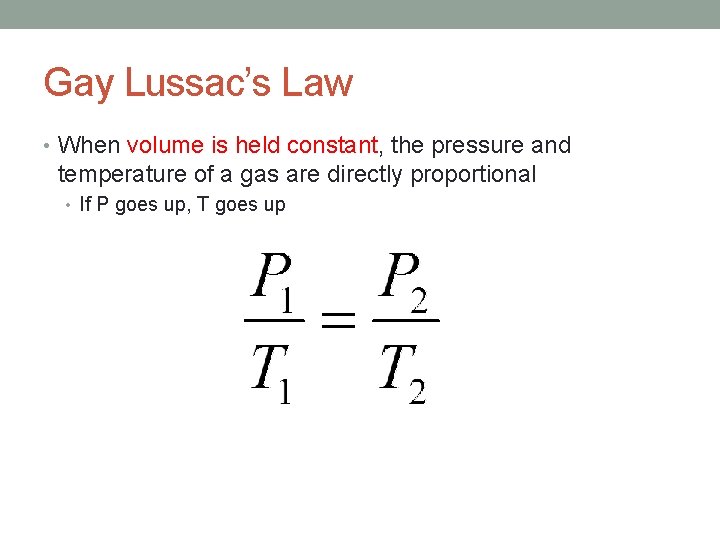
Gay Lussac’s Law • When volume is held constant, the pressure and temperature of a gas are directly proportional • If P goes up, T goes up

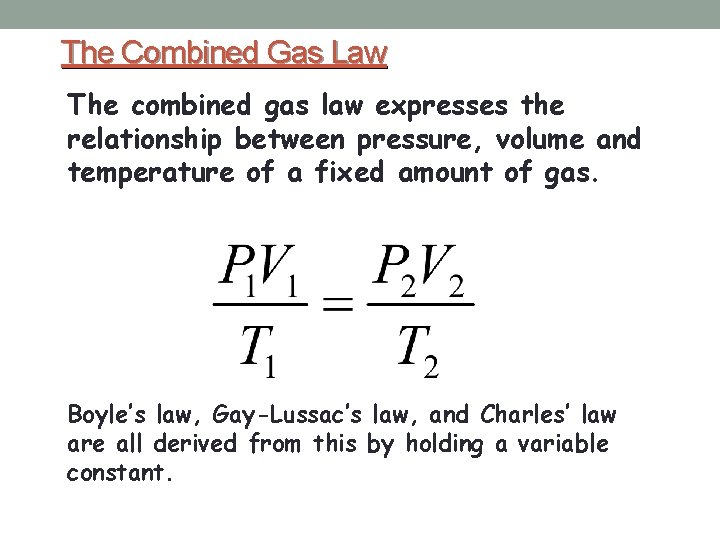
The Combined Gas Law The combined gas law expresses the relationship between pressure, volume and temperature of a fixed amount of gas. Boyle’s law, Gay-Lussac’s law, and Charles’ law are all derived from this by holding a variable constant.

Health Note When a scuba diver is several hundred feet under water, the high pressures cause N 2 from the tank air to dissolve in the blood. If the diver rises too fast, the dissolved N 2 will form bubbles in the blood, a dangerous and painful condition called "the bends". Helium, which is inert, less dense, and does not dissolve in the blood, is mixed with O 2 in scuba tanks used for deep descents.

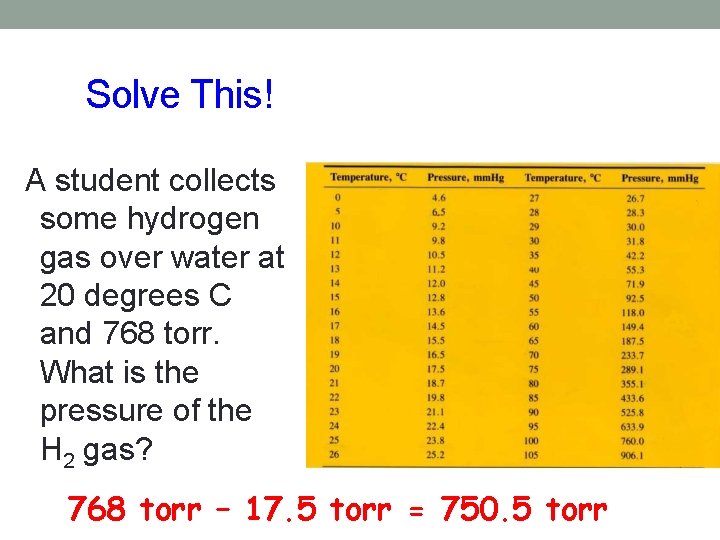
Dalton’s Law of Partial Pressures • Each gas exerts the same pressure it would if it alone was present at the same temperature • Gas collected over water- pressure in the container is the sum of the vapor pressure of the gas and the water’s vapor pressure • Subtract the water vapor pressure from the total pressure to obtain the pressure of the gas alone

Solve This! A student collects some hydrogen gas over water at 20 degrees C and 768 torr. What is the pressure of the H 2 gas? 768 torr – 17. 5 torr = 750. 5 torr

Ideal Gas Law PV = n. RT v. P = pressure in atm v. V = volume in liters vn = moles v. R = proportionality constant v= 0. 08206 L atm/ mol·K v. T = temperature in Kelvins Holds closely at P < 1 atm

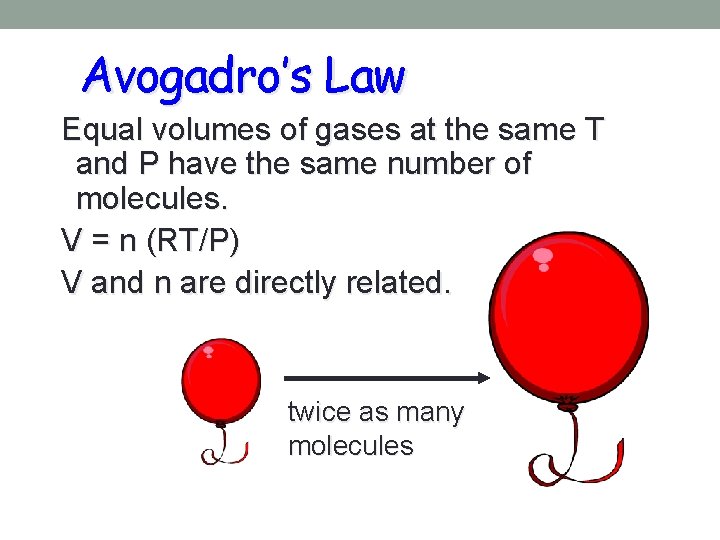
Avogadro’s Law • Equal volumes of different gases, at the same temp and pressure contain the same number of molecules • How would the number of molecules in 2 liters of hydrogen gas compare with the number of molecules in 2 liters of oxygen gas at the same temperature and pressure? • Why is 22. 4 liters called the molar volume of gas?

Avogadro’s Law Equal volumes of gases at the same T and P have the same number of molecules. V = n (RT/P) V and n are directly related. twice as many molecules

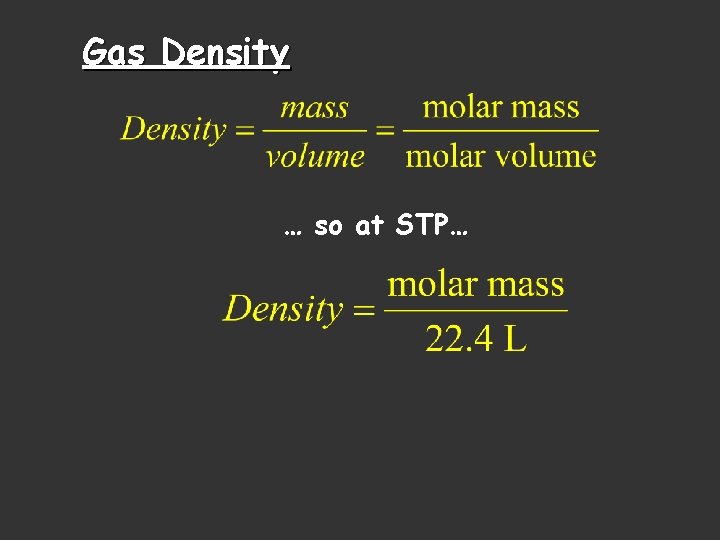
Gas Density … so at STP…

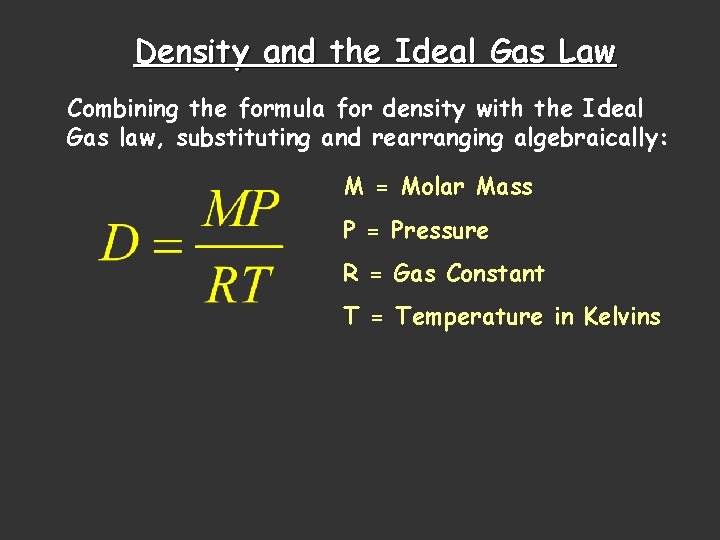
Density and the Ideal Gas Law Combining the formula for density with the Ideal Gas law, substituting and rearranging algebraically: M = Molar Mass P = Pressure R = Gas Constant T = Temperature in Kelvins
 Nature and nature's law lay hid in night
Nature and nature's law lay hid in night Useless laws weaken the necessary laws
Useless laws weaken the necessary laws The nature of gases
The nature of gases Module 3: topic 1 laws of nature
Module 3: topic 1 laws of nature Permanent facts examples
Permanent facts examples Module 3 topic 1 laws of nature
Module 3 topic 1 laws of nature Gas laws crash course
Gas laws crash course Do pressure and volume have a direct relationship
Do pressure and volume have a direct relationship Gas laws
Gas laws What is the combined gas law
What is the combined gas law Bourdon gauge gas law
Bourdon gauge gas law Different gas laws
Different gas laws Charles law
Charles law Gas law conceptual questions
Gas law conceptual questions Pressure and volume inversely proportional
Pressure and volume inversely proportional Chapter 14 the behavior of gases worksheet answer key
Chapter 14 the behavior of gases worksheet answer key 3 gas laws
3 gas laws Different gas laws
Different gas laws Combined gas law worksheet
Combined gas law worksheet 13-4 practice problems chemistry
13-4 practice problems chemistry Which gas laws are inversely proportional
Which gas laws are inversely proportional Charles law formula
Charles law formula