Gases Nature of Gases Gases have mass They









- Slides: 12

Gases

Nature of Gases • • Gases have mass They are easily compressed Gases fill their container completely Different gases can move through each other quite rapidly ( diffusion ) • Gases exert pressure • The pressure of a gas is dependent on the temperature.

Additional Properties • Expansion – spread out to fill their container • Fluidity – they flow • Low Density- particles are 1000 times farther apart in a gas than either a solid or a liquid • Compressibility – these particles can be pushed together • Diffusion – spontaneous mixing of two substances, by random movement • Effusion- passing through tiny holes, why He leaks out of a balloon

Kinetic-Molecular Theory • The Kinetic is used to explain why the gases behave the way they do. • It has about 6 assumptions

• Gases are made up of tiny winy itsi bitsy yellow poka dotted particles( atoms) • The particles are in constant motion, they never stop. • They have perfectly elastic collisions • They exert no force on one another, attraction or repulsion. • The average kinetic energy of a gas depends on the temperature Gases that do the above are called Ideal Gases

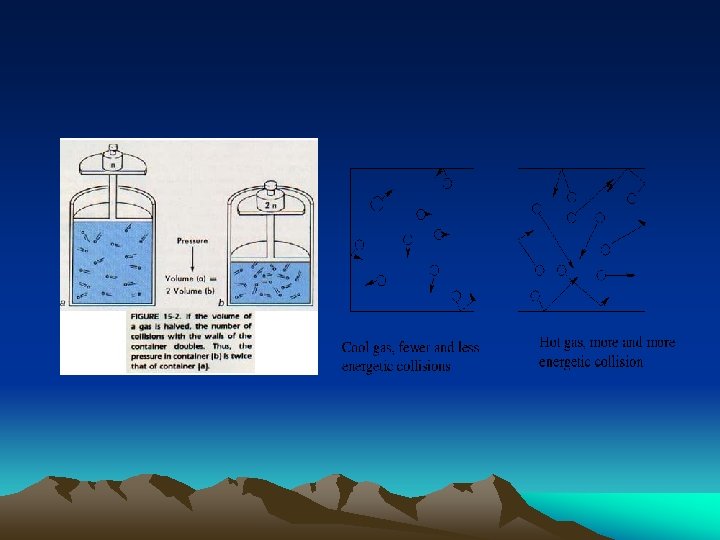
Pressure • pressure = force per unit area or force area Pressure is caused by the collisions of the particles with themselves and their container per unit time


• Any thing that changes the number of collision per unit time will change pressure. • Amount of gas • Size of container • temperature


Boyles law “J” tube

Measuring air pressure • Torricelli- the barometer

Units of air pressure • • All are standard pressure at sea level 760 mm. Hg 760 Torr 1 atm 101. 3 Kpa 14. 7 psi 1013 mb 29. 9 in.
 If your conditions are competitive we (place)
If your conditions are competitive we (place) It has 6 faces 12 edges 8 vertices
It has 6 faces 12 edges 8 vertices State boyle’s law.
State boyle’s law. Group 8 elements
Group 8 elements Nature and nature's laws lay hid in night
Nature and nature's laws lay hid in night Nature nature controversy
Nature nature controversy They perfect nature and are perfected by experience
They perfect nature and are perfected by experience What is mass audience
What is mass audience Rankings: what are they and do they matter?
Rankings: what are they and do they matter? They seek him here they seek him there
They seek him here they seek him there You are not rejected
You are not rejected Jordan 14
Jordan 14 Grammar rules frustrate me they're not logical they are so
Grammar rules frustrate me they're not logical they are so





















