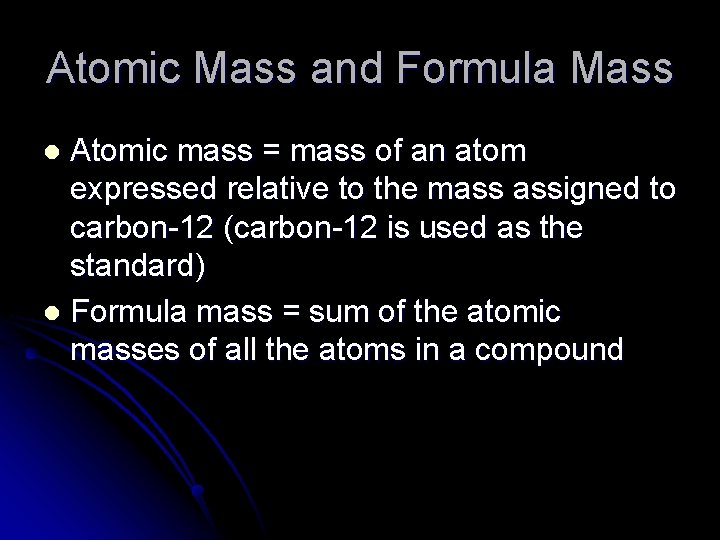
Atomic Mass and Formula Mass Atomic mass mass



















- Slides: 28

Atomic Mass and Formula Mass Atomic mass = mass of an atom expressed relative to the mass assigned to carbon-12 (carbon-12 is used as the standard) l Formula mass = sum of the atomic masses of all the atoms in a compound l

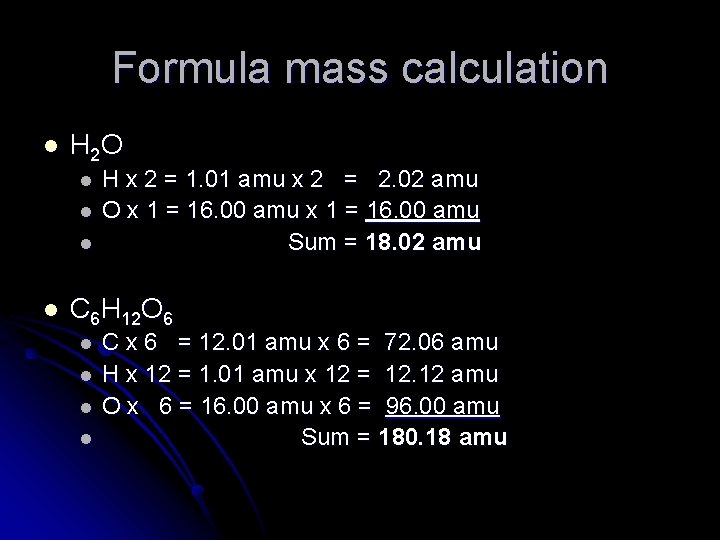
Formula mass calculation l H 2 O l l H x 2 = 1. 01 amu x 2 = 2. 02 amu O x 1 = 16. 00 amu Sum = 18. 02 amu C 6 H 12 O 6 l l C x 6 = 12. 01 amu x 6 = 72. 06 amu H x 12 = 1. 01 amu x 12 = 12. 12 amu O x 6 = 16. 00 amu x 6 = 96. 00 amu Sum = 180. 18 amu

Measuring Matter l Counting Units l 12 = dozen l 2 = pair l 500 = ream This works for objects we can manipulate with bare hands. l Chemists need a way to accurately count the number of atoms, molecules, or formula units in a sample. l

The Mole!!!!!!! SI base unit for measuring the amount of a substance l Mole = number of carbon atoms in exactly 12 grams of pure carbon-12. l l Carbon-12 used as a standard. = 6. 02 X 1023 representative particles l 1 mole anything l Representative particles can be: l Atoms l Molecules (Covalently bonded) l Formula units (Ionically bonded)

l Atoms, molecules, and formula units are so small it’s impossible to count them individually. l So, chemists need a special counting unit.

Avogadro’s Number l Avogadro’s # = 6. 02 X 1023 l 60200000000000 l You don’t have to write this number, it’s just for effect…just know it’s a big number and use the scientific notation always

So how big is Avogadro’s # ? If you gave away 1 million dollars to each person on Earth every day, it would take more than 3000 years to distribute the money. l If you spread Avogadro's number of unpopped popcorn kernels across the USA, the entire country would be covered in popcorn to a depth of over 9 miles. l

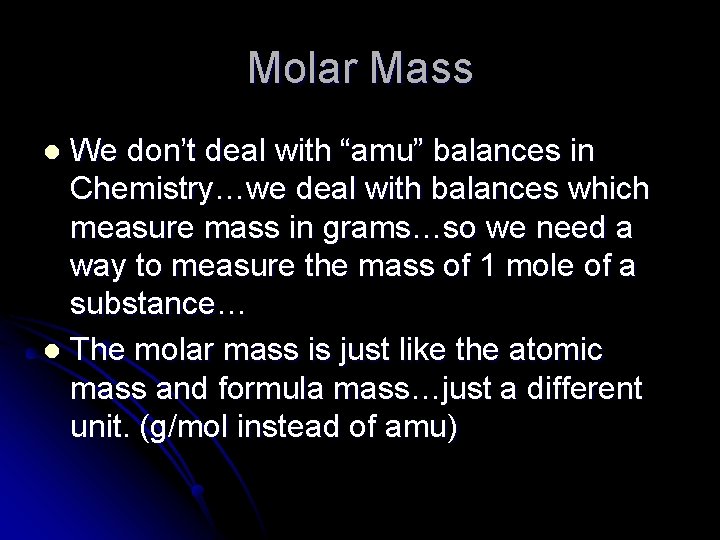
Molar Mass We don’t deal with “amu” balances in Chemistry…we deal with balances which measure mass in grams…so we need a way to measure the mass of 1 mole of a substance… l The molar mass is just like the atomic mass and formula mass…just a different unit. (g/mol instead of amu) l

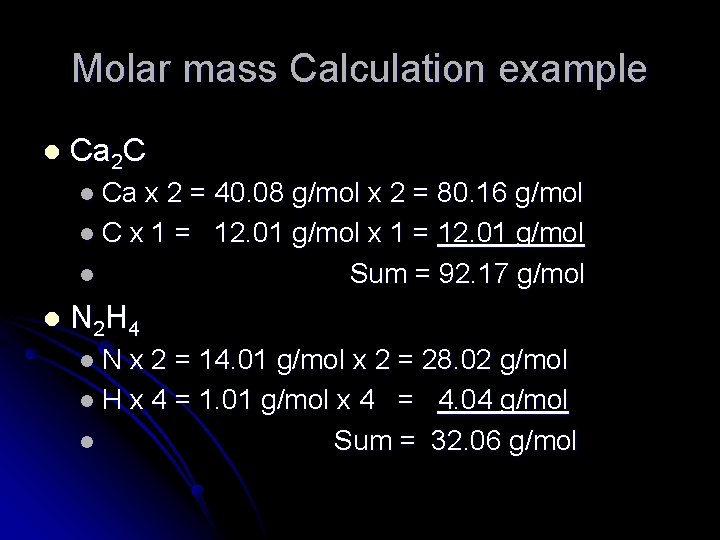
Molar mass Calculation example l Ca 2 C l Ca x 2 = 40. 08 g/mol x 2 = 80. 16 g/mol l C x 1 = 12. 01 g/mol l Sum = 92. 17 g/mol l N 2 H 4 l. N x 2 = 14. 01 g/mol x 2 = 28. 02 g/mol l H x 4 = 1. 01 g/mol x 4 = 4. 04 g/mol l Sum = 32. 06 g/mol

Watch out for these… l Let’s say you have 1 mole of Ca. Cl 2 … l How many moles of Ca 2+ do you have? l How many moles of Cl 1 - do you have? l How many formula units of Ca. Cl 2 do you have?

Mass and the Mole l 1 mole of Fe has the same number of atoms as 1 mole of Ne l 6. 02 l X 10 23 atoms of each 1 mole of Fe does not have the same mass as 1 mole of Ne l 1 atom of Fe does not have the same mass as 1 atom of Ne

Mass to Mole Example l How many moles are present in 4. 395 grams of sodium chloride?

Mole to Mass Example l Suppose you have a 0. 548 mole sample of dinitrogen triiodide. What is the mass (in grams) of this sample?

Moles to Particles Example l A sample of potassium chloride contains 2. 57 moles. How many formula units of potassium chloride are present in this sample?

Particles to Moles Example l A sample of chlorine gas contains 5. 48 X 1024 molecules of chlorine gas. How many moles of chlorine gas does this sample contain?

Multistep Conversions The mole is the key here. It is the thing which chemists use the most when dealing with calculations involving chemical reactions. l There are two main types of multipstep conversions: l l Mass to particle l Particle to mass

Mass to Particle Example l A baker needs 758 grams of sucrose (C 12 H 22 O 11) to bake a cake. How many sucrose molecules will be in the unbaked cake?

Particles to Mass Example l 8. 0 X 1020 atoms of calcium are present in a multivitamin. How many grams of calcium does a person consume when they take the vitamin?

Moles and Gases At the same temperature and pressure, equal volumes of gases contain the same number of gas particles. l STP = Standard Temperature and Pressure (0 o. C and 1 atmosphere) l At STP, it was found experimentally that 1 mole of any gas had a volume of 22. 4 L. l We call this volume the molar volume l

Moles to Volume l A chemical reaction produces 0. 82 moles of oxygen gas. What volume will that gas occupy assuming the chemical reaction occurred at STP?

Volume to Moles l A huge latex balloon with a volume of 3675 L contains how many moles of helium at STP?

Multistep Conversions using Molar Volume l How many grams of oxygen gas are present in a sample of oxygen gas with a volume of 58. 4 L at STP? l A sample of neon gas has a volume of 5. 2 L at STP. How many grams of neon are present in the sample? How many atoms of neon are present in the sample?

Percent Composition The mass of each element in a compound compared to the entire mass of the compound, and multiplied by 100%. l (Part/Whole) X 100% l l This l needs to be done for each element! Two ways to determine percent composition l Given a chemical formula l Given experimental mass data

% Composition Given Chemical Formula l Determine the percent composition for the following chemical compounds: l C 6 H 12 O 6 l (NH 4)2 S

% Composition Given Mass Data If a compound has a total mass of 4. 50 grams, and contains 1. 25 grams of carbon and 3. 25 grams of oxygen, what is its percent composition? l If a compound contains only 3. 10 grams of iron and 1. 25 grams of oxygen, what is its percent composition? l

Empirical Formulas that give the simplest-whole number ratio of the atoms of the elements in a compound. l These cannot be reduced any further! l Example: Ca. Cl 2 cannot be reduced. l Example: CH 2 O cannot be reduced. l Example: Ba(OH)2 cannot be reduced. l

Molecular Formulas The formula that gives the actual number of atoms of each element in a molecular compound. l This formula is always a whole-number multiple of the empirical formula. l This formula can be reduced to yield the empirical formula. l Example: C 6 H 12 O 6 can be reduced to CH 2 O l

Determining Molecular Formula From Empirical Formula Beta carotene can be broken down to form vitamin A. The empirical formula for beta carotene is C 5 H 7. The molar mass of beta carotene is 536 g/mol. What is the molecular formula? l The molar mass of TNT is 227. 0 g/mole. If its empirical formula was determined to be C 7 H 5 N 3 O 6 What is its molecular formula? l