Starter S146 List five properties of gases Chapter













- Slides: 29

Starter S-146 List five properties of gases.

Chapter 14 The Behavior of Gases

Chapter 14 14. 1 Properties of Gases

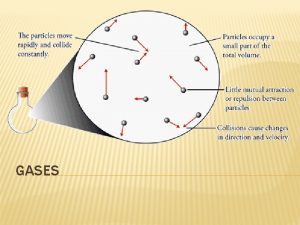
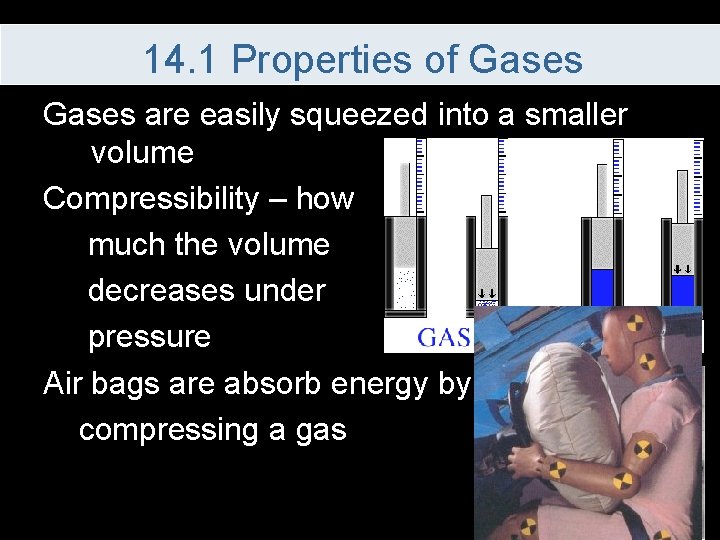
14. 1 Properties of Gases are easily squeezed into a smaller volume Compressibility – how much the volume decreases under pressure Air bags are absorb energy by compressing a gas

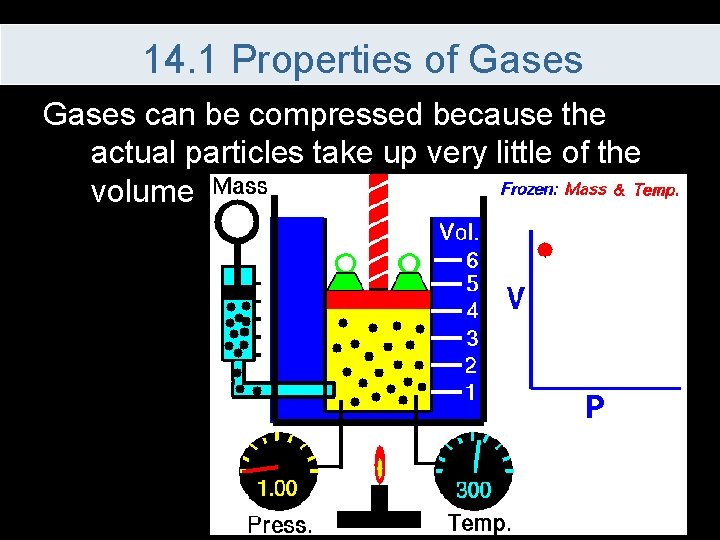
14. 1 Properties of Gases can be compressed because the actual particles take up very little of the volume

14. 1 Properties of Gases Four variables are used to describe gases 1. Pressure (P) – force per unit area on the container 2. Volume (V) – in liters, how much space the gas take up 3. Temperature (T) – average kinetic energy 4. Number of Moles (n) – how much of the gas is present

Chapter 14 14. 2 The Gas Laws

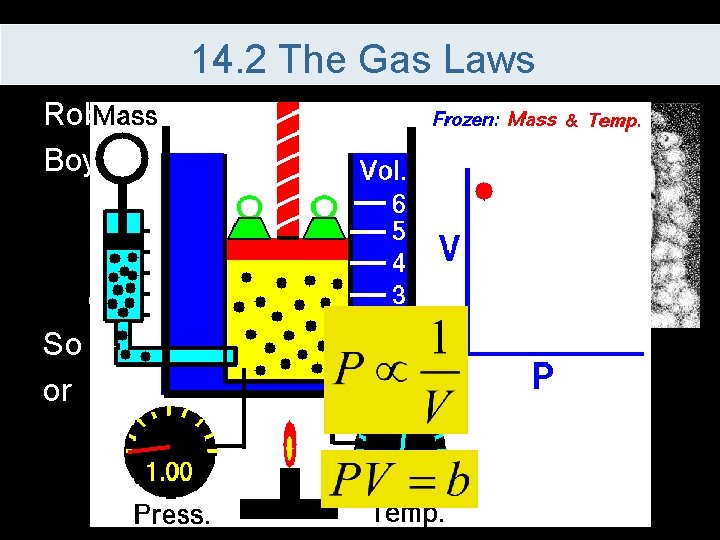
14. 2 The Gas Laws Robert Boyle’s Law – If temperature is kept constant, as pressure increases the volume decreases So if T is constant or

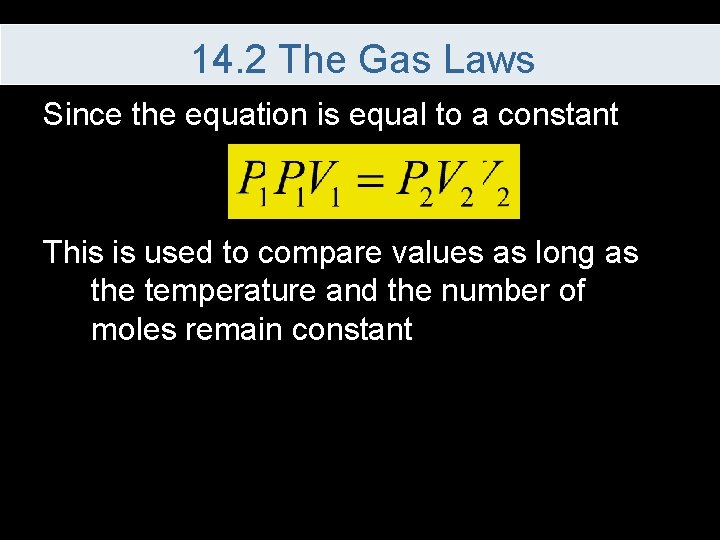
14. 2 The Gas Laws Since the equation is equal to a constant This is used to compare values as long as the temperature and the number of moles remain constant

14. 2 The Gas Laws Jacques Charles’s Law – If pressure is kept constant, at temperature increases, pressure also increases That is or

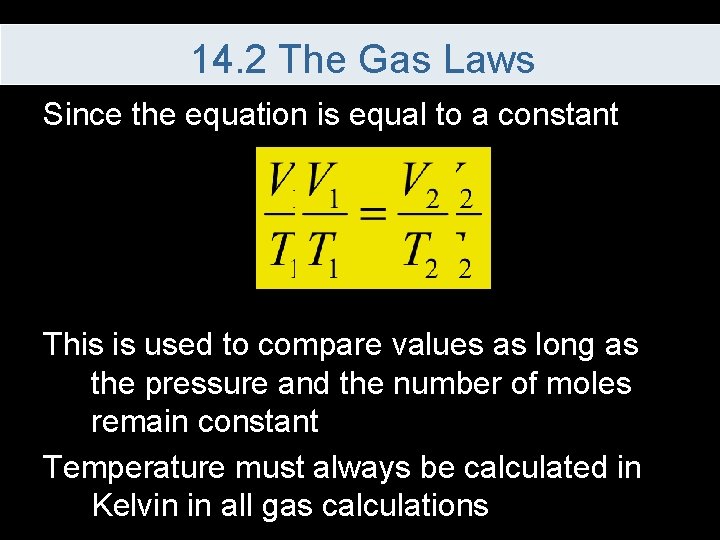
14. 2 The Gas Laws Since the equation is equal to a constant This is used to compare values as long as the pressure and the number of moles remain constant Temperature must always be calculated in Kelvin in all gas calculations

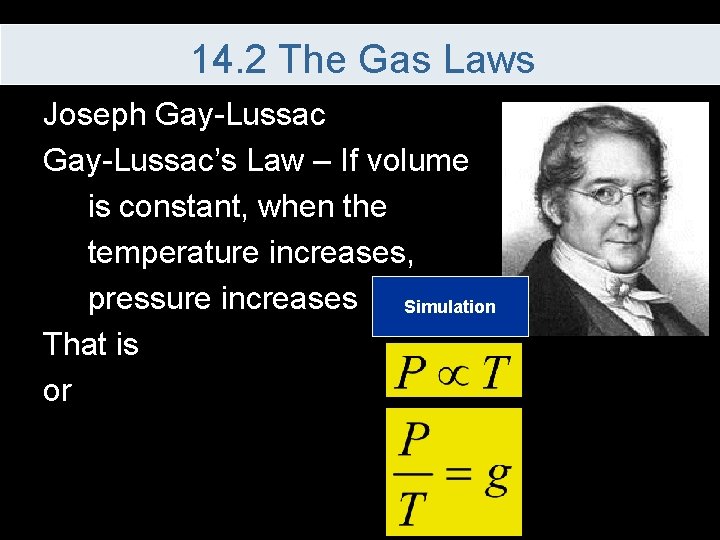
14. 2 The Gas Laws Joseph Gay-Lussac’s Law – If volume is constant, when the temperature increases, pressure increases Simulation That is or

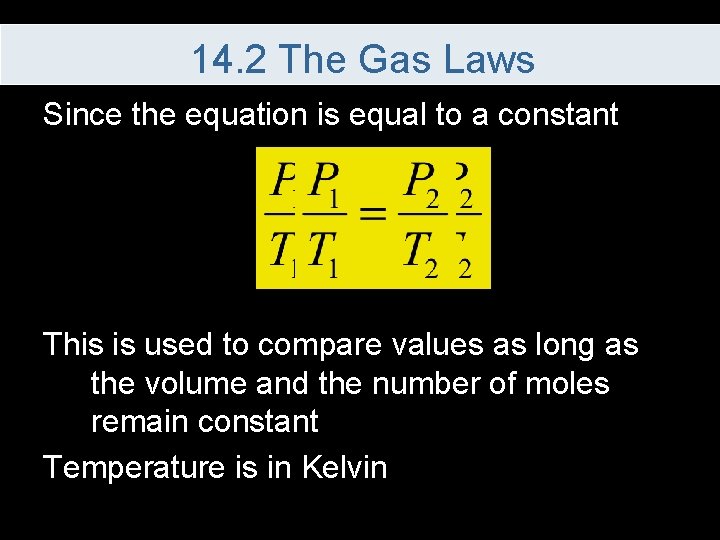
14. 2 The Gas Laws Since the equation is equal to a constant This is used to compare values as long as the volume and the number of moles remain constant Temperature is in Kelvin

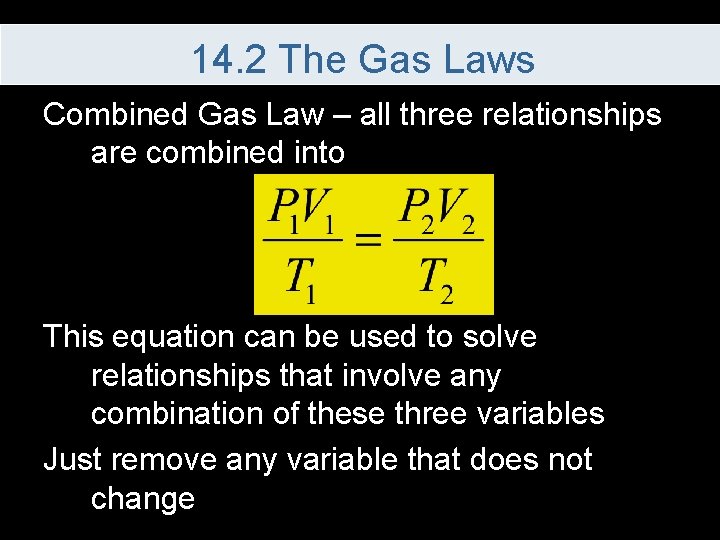
14. 2 The Gas Laws Combined Gas Law – all three relationships are combined into This equation can be used to solve relationships that involve any combination of these three variables Just remove any variable that does not change

14. 2 The Gas Laws Example – a sample of oxygen gas at STP is heated to 50. 0 o. C at a constant volume, what is the new pressure? What stays constant?

14. 2 The Gas Laws Example – If 40. 0 m. L of nitrogen gas at 812 mm. Hg and 75. 0 o. C is cooled to -30. 0 o. C and has the pressure reduced to 125 mm. Hg, what is the new volume?

Chapter 14 14. 3 Ideal Gas

14. 3 Ideal Gas Avogadro’s Law – if the temperature and pressure are held constant, an increase in gas particles (moles) will cause an increase in the volume Simulation This can be written as

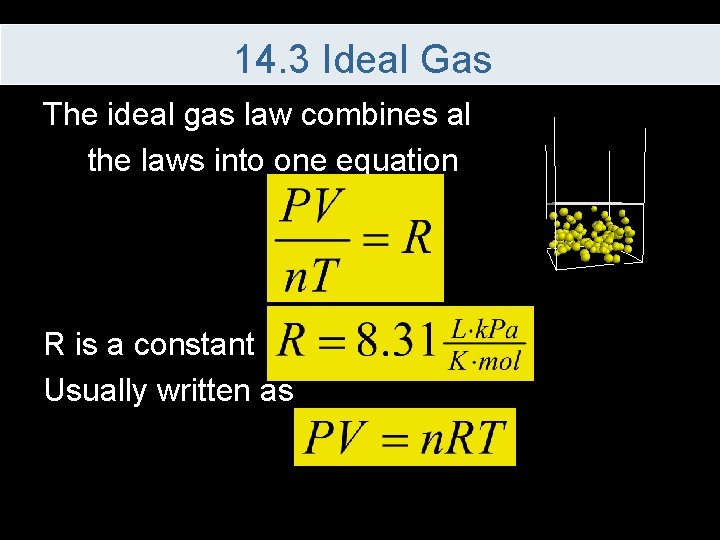
14. 3 Ideal Gas The ideal gas law combines all the laws into one equation R is a constant Usually written as

14. 3 Ideal Gas A container has 2, 240, 000 L of methane gas (CH 4) at a pressure of 1500 k. Pa and a temperature of 42 o. C. A. How many moles of gas are in the container? B. How many grams of gas are in the container?

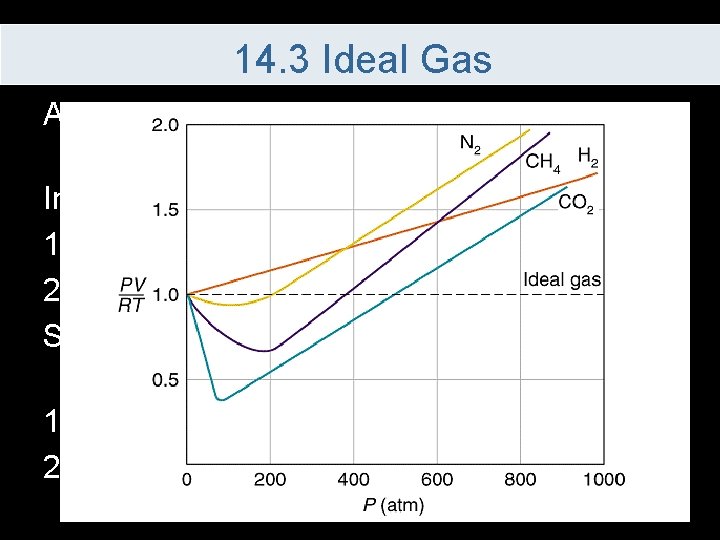
14. 3 Ideal Gas An ideal gas – is an imaginary gas that always follows the ideal gas law In a real gas 1. Particles take up volume 2. Force exist between particles So real gases deviate from the an ideal gas especially at 1. Low temperature 2. High pressure

Chapter 14 14. 4 Gases: Mixtures and Movements

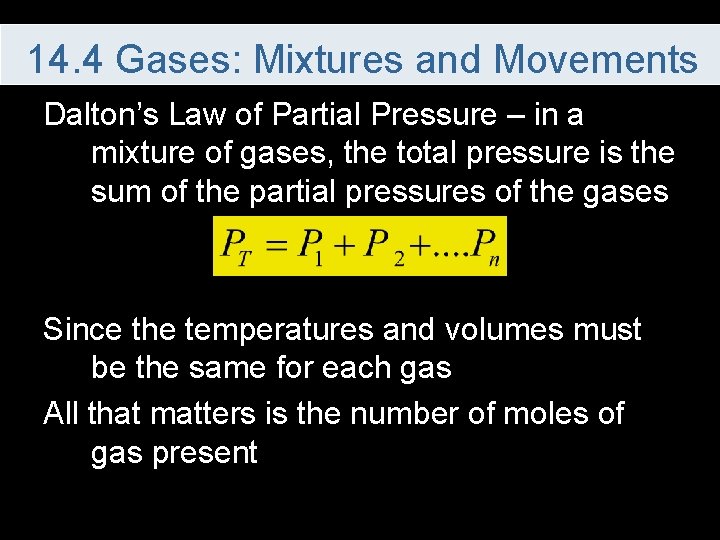
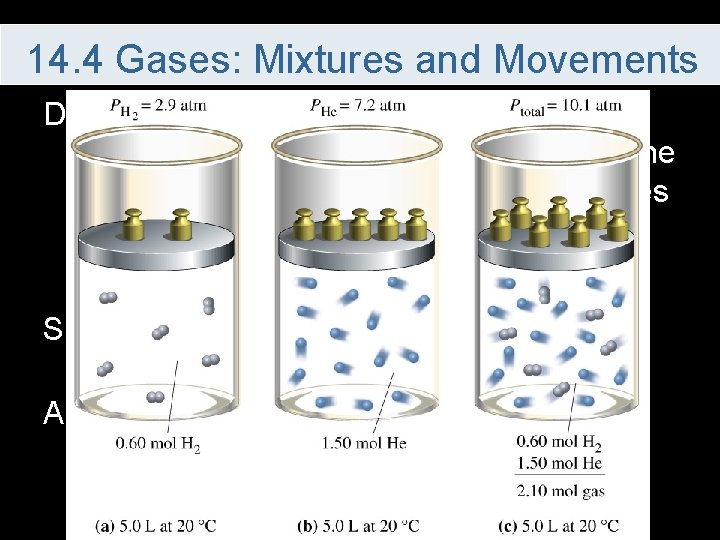
14. 4 Gases: Mixtures and Movements Dalton’s Law of Partial Pressure – in a mixture of gases, the total pressure is the sum of the partial pressures of the gases Since the temperatures and volumes must be the same for each gas All that matters is the number of moles of gas present

14. 4 Gases: Mixtures and Movements Dalton’s Law of Partial Pressure – in a mixture of gases, the total pressure is the sum of the partial pressures of the gases Since the temperatures and volumes must be the same for each gas All that matters is the number of moles of gas present

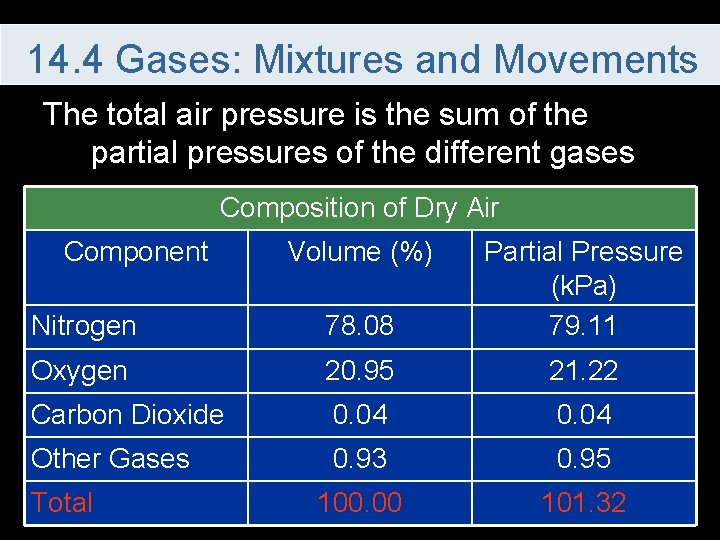
14. 4 Gases: Mixtures and Movements The total air pressure is the sum of the partial pressures of the different gases Composition of Dry Air Component Nitrogen 78. 08 Partial Pressure (k. Pa) 79. 11 Oxygen 20. 95 21. 22 Carbon Dioxide 0. 04 Other Gases 0. 93 0. 95 100. 00 101. 32 Total Volume (%)

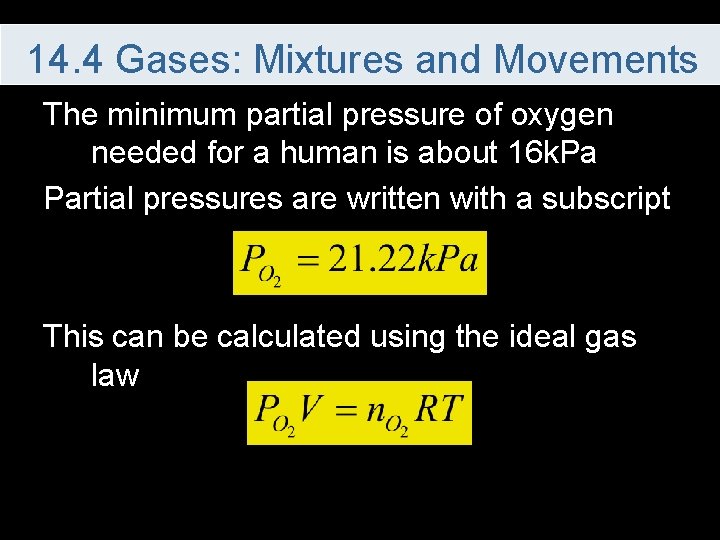
14. 4 Gases: Mixtures and Movements The minimum partial pressure of oxygen needed for a human is about 16 k. Pa Partial pressures are written with a subscript This can be calculated using the ideal gas law

14. 4 Gases: Mixtures and Movements Thomas Graham Diffusion – tendency of particles to move toward areas of lower concentration Effusion - is the process in which individual molecules flow through a hole without collisions between molecules Simulation

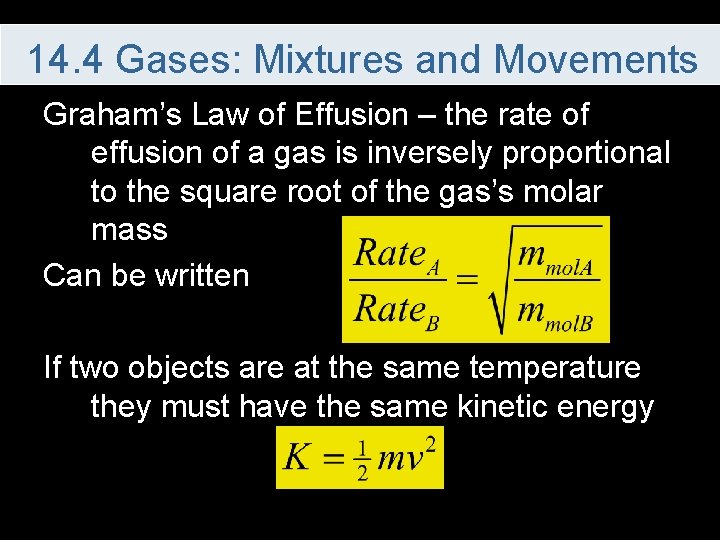
14. 4 Gases: Mixtures and Movements Graham’s Law of Effusion – the rate of effusion of a gas is inversely proportional to the square root of the gas’s molar mass Can be written If two objects are at the same temperature they must have the same kinetic energy

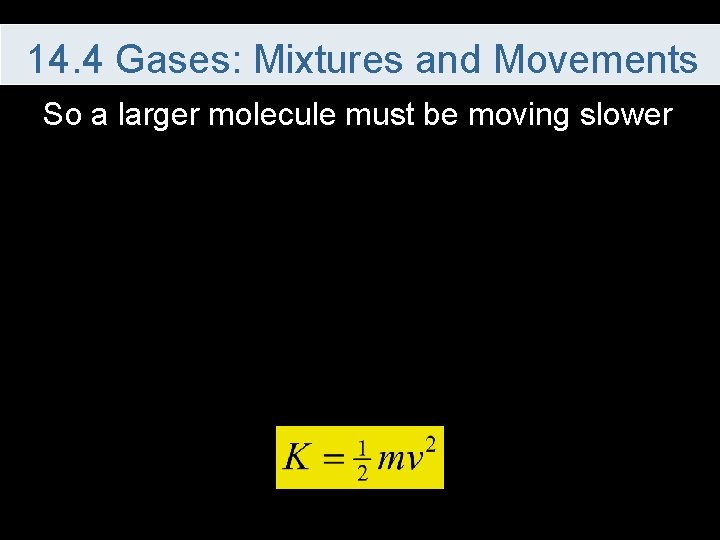
14. 4 Gases: Mixtures and Movements So a larger molecule must be moving slower
 List 2 of the important properties of gases
List 2 of the important properties of gases Buoyancyability
Buoyancyability Solid liquid and gas particles
Solid liquid and gas particles General properties of gases
General properties of gases Four properties of gas
Four properties of gas 5 properties of gases
5 properties of gases Properties of gases
Properties of gases Properties of noble gas
Properties of noble gas Physical properties of gases
Physical properties of gases Gases have low densities
Gases have low densities Why was the term inert gases dropped?
Why was the term inert gases dropped? Properties of solid liquid and gas
Properties of solid liquid and gas Charles's law
Charles's law Properties of gases
Properties of gases List the properties of x radiation chapter 38
List the properties of x radiation chapter 38 Five years have past five summers with the length
Five years have past five summers with the length Five of five
Five of five 5 senses and 5 elements
5 senses and 5 elements Macbeth act five scene five
Macbeth act five scene five Chapter 11 review gases section 1
Chapter 11 review gases section 1 Chapter 11 review gases section 1
Chapter 11 review gases section 1 Gas laws formula
Gas laws formula Chapter 14 behavior of gases
Chapter 14 behavior of gases Section 13.2 the combined gas law and avogadro's principle
Section 13.2 the combined gas law and avogadro's principle Chapter 14 solids liquids and gases worksheet answers
Chapter 14 solids liquids and gases worksheet answers Kinetic molecular theory of liquids and solids
Kinetic molecular theory of liquids and solids List five drama personnel
List five drama personnel Solomon wise decision
Solomon wise decision Hair design
Hair design Foundations u disc profile
Foundations u disc profile