Stoichiometry The quantitative study of reactants and products


- Slides: 15

Stoichiometry The quantitative study of reactants and products in a chemical reaction

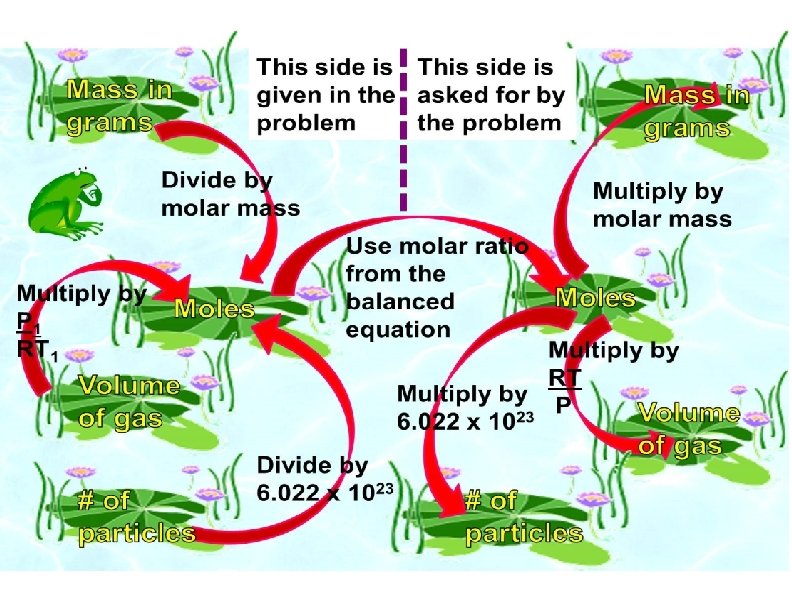
Stoichiometry • Whether the units given for reactants or products are moles , grams , liters (for gases), or some other units, we use moles to calculate the amount of product formed in a reaction


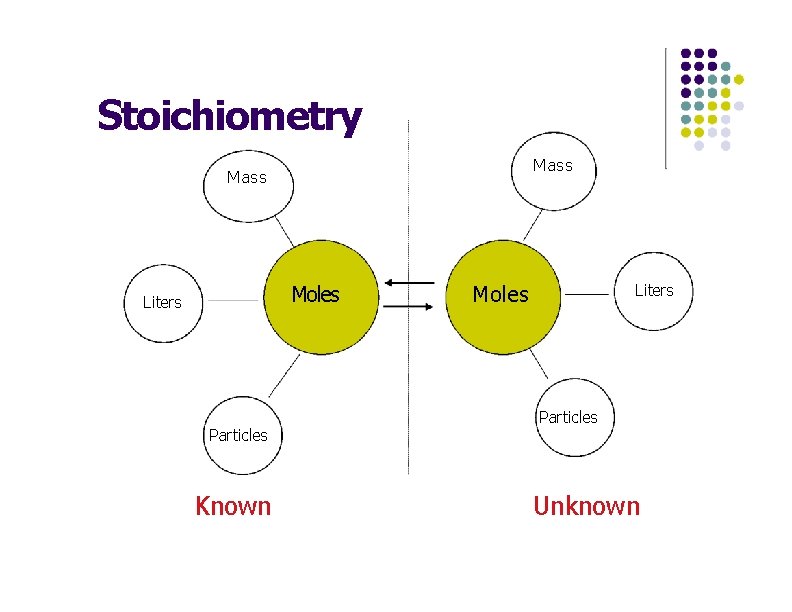
Stoichiometry Mass Moles Liters Particles Known Moles Liters Particles Unknown

Review before starting · Dimensional Analysis · Conversion Factors · The Mole · Molar Conversions · Balancing Chemical Equations

Stoichiometry Problem Types · · · Mole to Mass & Mass to Mole Mass to Mass Volume to Moles or Mass Limiting Reactants & Per Cent Yield

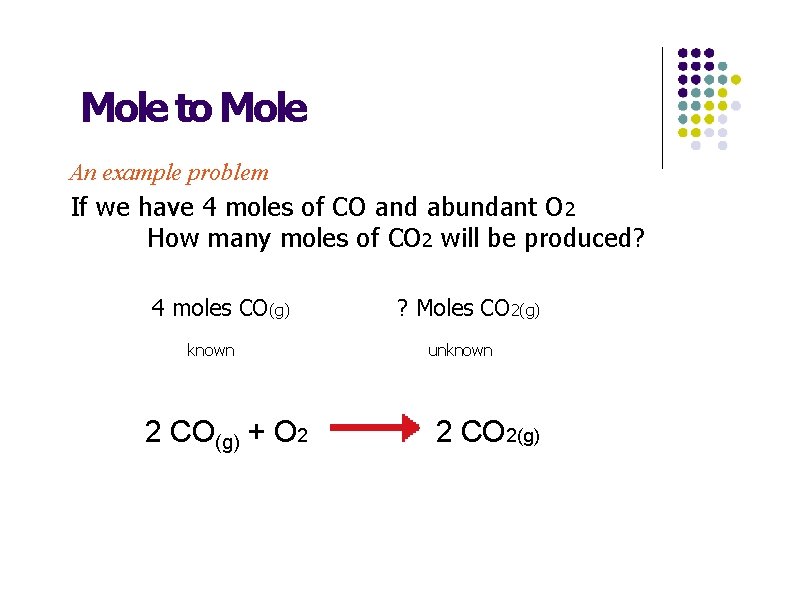
Mole to Mole An example problem If we have 4 moles of CO and abundant O 2 How many moles of CO 2 will be produced? 4 moles CO(g) known 2 CO(g) + O 2 ? Moles CO 2(g) unknown 2 CO 2(g)

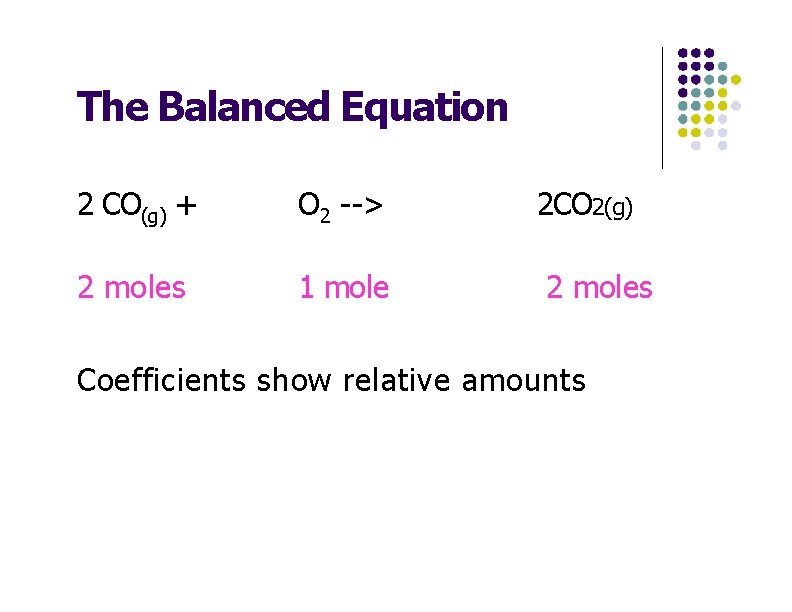
The Balanced Equation 2 CO(g) + O 2 --> 2 moles 1 mole 2 CO 2(g) 2 moles Coefficients show relative amounts

Limiting Reagents To make a dozen brownies the recipe calls for 2 cups flour, 112 grams chocolate, 25 O ml water. You have 2 cups flour 50 grams chocolate, & 250 ml water If you want to make quality brownies you will make less than a dozen and have flour & water left over! What is the limiting reagent ?

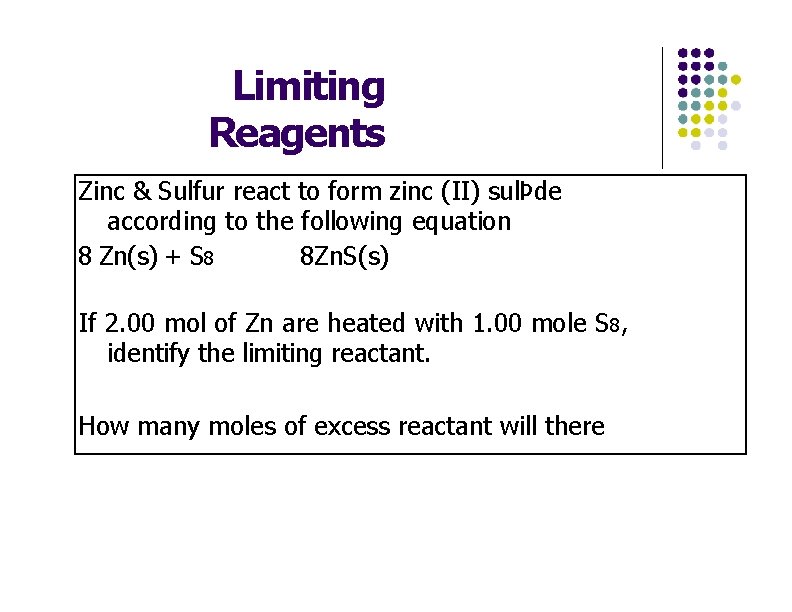
Limiting Reagents Zinc & Sulfur react to form zinc (II) sulÞde according to the following equation 8 Zn(s) + S 8 8 Zn. S(s) If 2. 00 mol of Zn are heated with 1. 00 mole S 8, identify the limiting reactant. How many moles of excess reactant will there

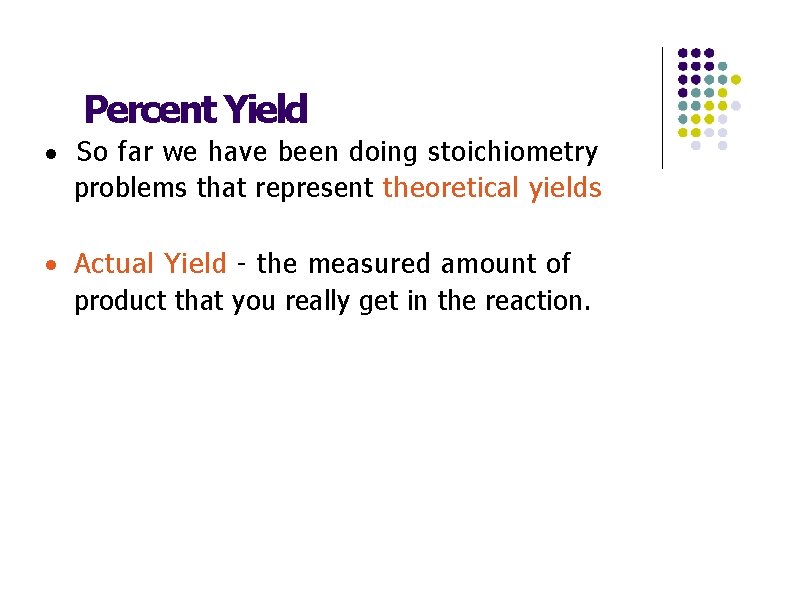
Percent Yield · So far we have been doing stoichiometry problems that represent theoretical yields · Actual Yield - the measured amount of product that you really get in the reaction.

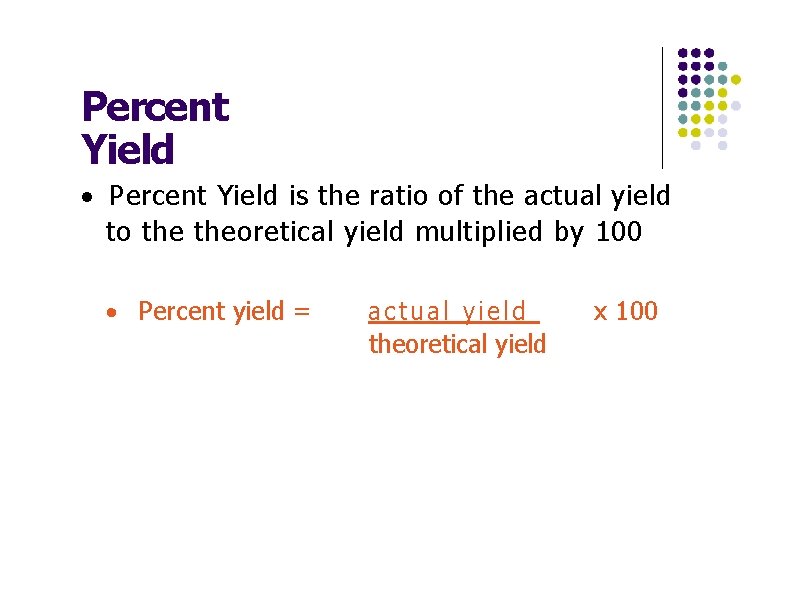
Percent Yield · Percent Yield is the ratio of the actual yield to theoretical yield multiplied by 100 · Percent yield = actual yield theoretical yield x 100

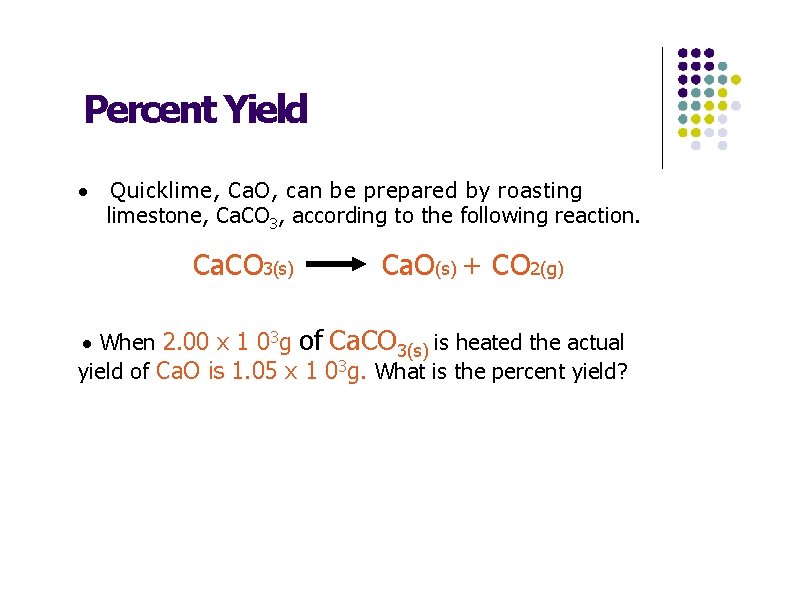
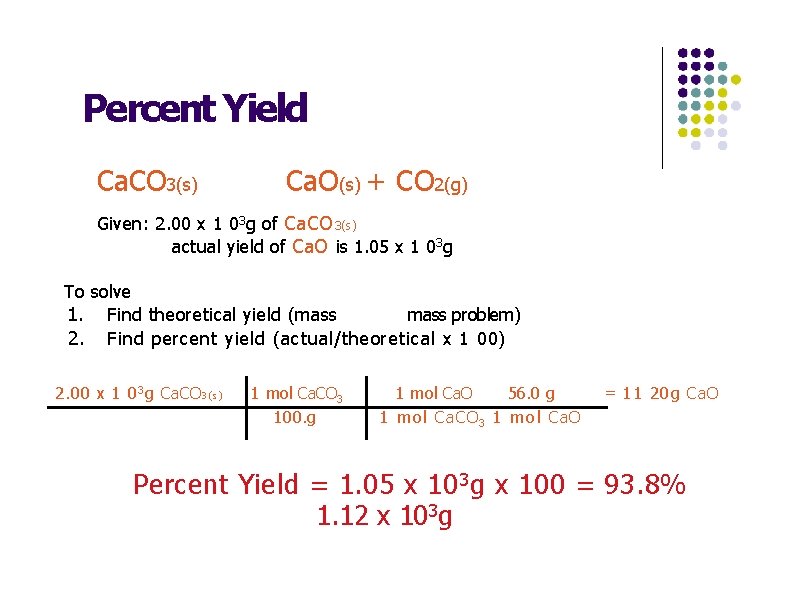
Percent Yield · Quicklime, Ca. O, can be prepared by roasting limestone, Ca. CO 3, according to the following reaction. Ca. CO 3(s) Ca. O(s) + CO 2(g) · When 2. 00 x 1 03 g of Ca. CO 3(s) is heated the actual yield of Ca. O is 1. 05 x 1 03 g. What is the percent yield?

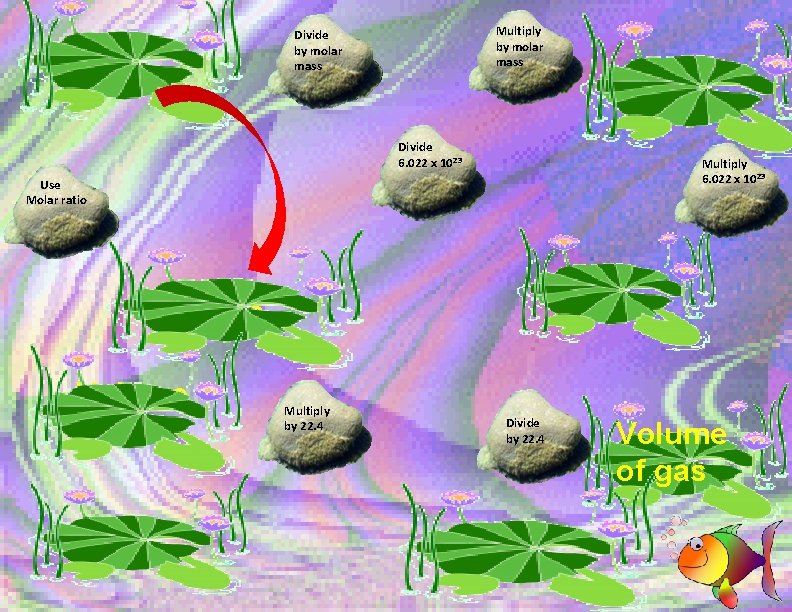
Multiply by molar mass Divide 6. 022 x 10 23 Multiply 6. 022 x 10 23 Use Molar ratio Moles Volume of gas Multiply by 22. 4 Divide by 22. 4 Volume of gas

Percent Yield Ca. CO 3(s) Ca. O(s) + CO 2(g) Given: 2. 00 x 1 03 g of Ca. CO 3(s) actual yield of Ca. O is 1. 05 x 1 03 g To solve 1. Find theoretical yield (mass problem) 2. Find percent yield (actual/theoretical x 1 00) 2. 00 x 1 0 3 g Ca. CO 3(s) 1 mol Ca. CO 3 100. g 1 mol Ca. O 56. 0 g 1 mol Ca. CO 3 1 mol Ca. O = 11 20 g Ca. O Percent Yield = 1. 05 x 10 3 g x 100 = 93. 8% 1. 12 x 103 g