Reaction Stoichiometry Chapter 9 Reaction Stoichiometry n Reaction














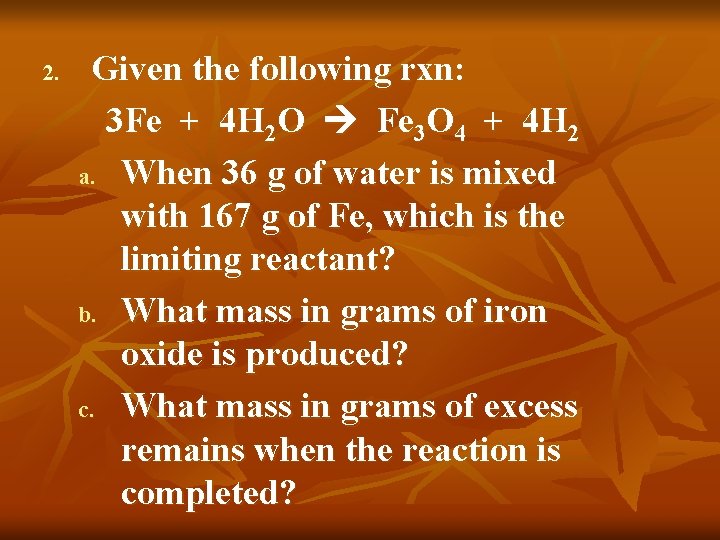




- Slides: 21

Reaction Stoichiometry Chapter 9

Reaction Stoichiometry n Reaction stoichiometry – calculations of amounts of reactants and products of a chemical reaction

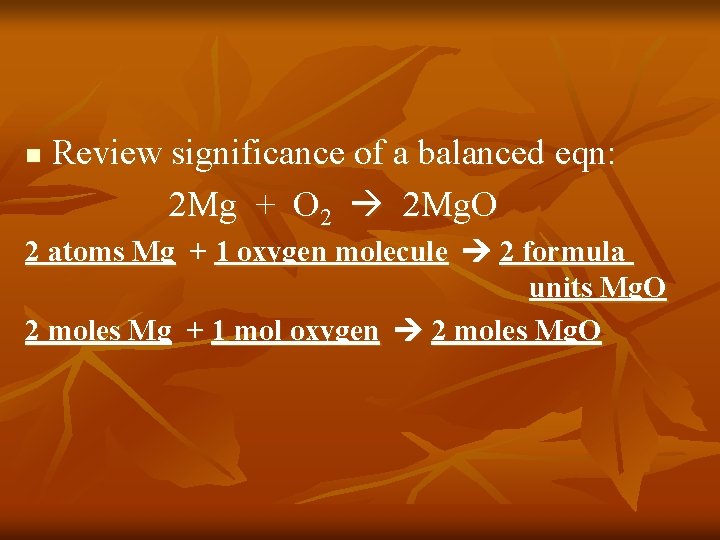
n Review significance of a balanced eqn: 2 Mg + O 2 2 Mg. O 2 atoms Mg + 1 oxygen molecule 2 formula units Mg. O 2 moles Mg + 1 mol oxygen 2 moles Mg. O

Since we can’t count out individual atoms, molecules or formula units, we will count the coefficients in mole units.

n Mole ratios: a conversion factor that relates the amounts of two different substances based on the balanced eqn. n Ex: 2 mol Mg. O 1 mol O 2 2 mol Mg. O

Molar Mass n n Review of Molar Mass: mass of one mole of substance. The molar mass of H 2 O is 18. 0 g. It can be expressed as 1 mol H 2 O 18 g H 2 O

Express the molar mass of the following in conversion factor form: a) Al 2 O 3 b) C 12 H 22 O 11 Section Review p 277

Stoichiometric Calculations Steps to Solving Stoichiometry Problems: 1. Balanced Eqn. Write given and unknown under eqn. 2. Convert given to moles 3. Mole Ratio: unknown/known 4. Covert to unit needed.

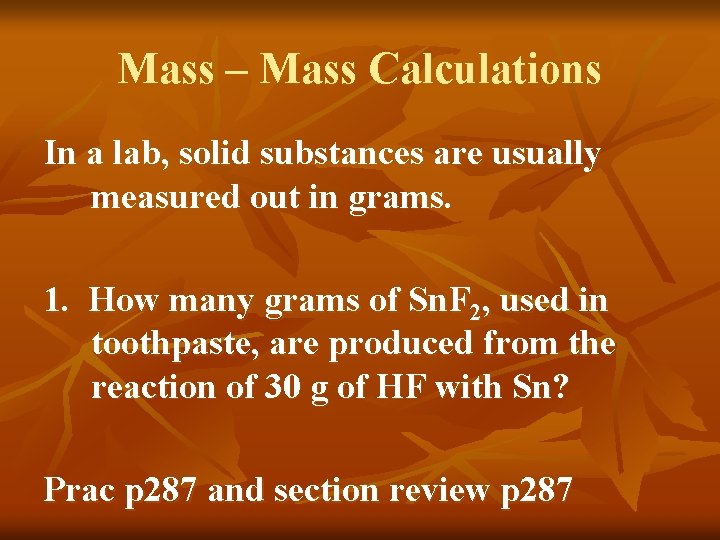
Mass – Mass Calculations In a lab, solid substances are usually measured out in grams. 1. How many grams of Sn. F 2, used in toothpaste, are produced from the reaction of 30 g of HF with Sn? Prac p 287 and section review p 287

Cu + Ag. NO 3 Cu(NO 3)2 + Ag 2. a. b. How many grams of silver will be produced if 125 g of copper is reacted with silver nitrate. How many grams of copper is needed to complete the rxn if 200 g of silver nitrate is used?

3. A camping lantern uses the reaction of calcium carbide, Ca. C 2 (s), and water to produce acetylene gas, C 2 H 2(g), and calcium hydroxide, solid. How many grams of water are required to produce 1. 55 moles of acetylene gas?

n Prac Problems p 282 -284.

Limiting Reactants n n Limiting reactant – reactant that runs out first and limits the amount of product produced. Excess reactant – the substance that is not used up in a rxn.

Strategy 1. Take each reactant amount and calculate the amount of one product it will make. The reactant that makes the smallest amount of product is the limiting reactant. The other is the excess reactant. 2. Calculate amount of excess reactant needed based on the limiting reactant quantity. 3. Determine amounts of products based on limiting reactant amt.

1. If 1. 21 moles of solid zinc are added to 2. 65 moles of hydrochloric acid, HCl, then zinc chloride, Zn. Cl 2 (aq), and hydrogen gas are formed. Determine which reactant is in excess and by what amount and calculate the number of moles of each product.

Step 1: Determining limiting reactant. Step 2: Determining amt of excess reactant needed. Step 3: Determining amt of rest of products.
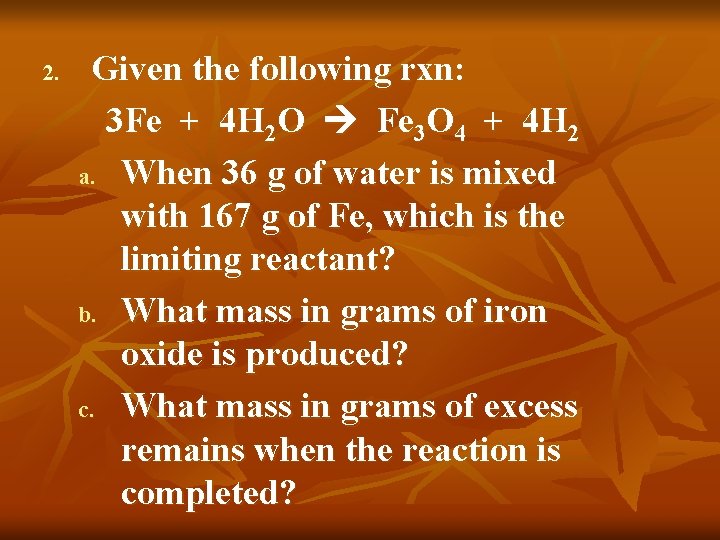
2. Given the following rxn: 3 Fe + 4 H 2 O Fe 3 O 4 + 4 H 2 a. When 36 g of water is mixed with 167 g of Fe, which is the limiting reactant? b. What mass in grams of iron oxide is produced? c. What mass in grams of excess remains when the reaction is completed?

n n Do prob on p 289 prac #1 and #2. The sample problem 9 -7 p 290. More practice on p 291

Percent Yield n Percent yield = actual yield * 100 theoretical yield Actual yield = amt of product obtain from experiment Theoretical yield = maximum amt of product can be produced according to calculations.

n Read sample prob 9 -8 p 293, then set up bal eqn and the given info and the unknown: C 6 H 6 + Cl 2 C 6 H 5 Cl + HCl 38. 8 g excess 38. 8 g actual 38. 8 g ? g theoretical A x 100 % yield of C 6 H 5 Cl T Prac p 294 and section review p 294

Given the following equation: C 6 H 6 + Cl 2 C 6 H 5 Cl + HCl When 36. 8 g of C 6 H 6 react with an excess of chlorine, the actual yield of C 6 H 5 Cl is 38. 8 g. What is the percent yield of C 6 H 5 Cl ? 1.