Chemical Reactions Types of Reactions and Reaction Symbols
Chemical Reactions Types of Reactions and Reaction Symbols
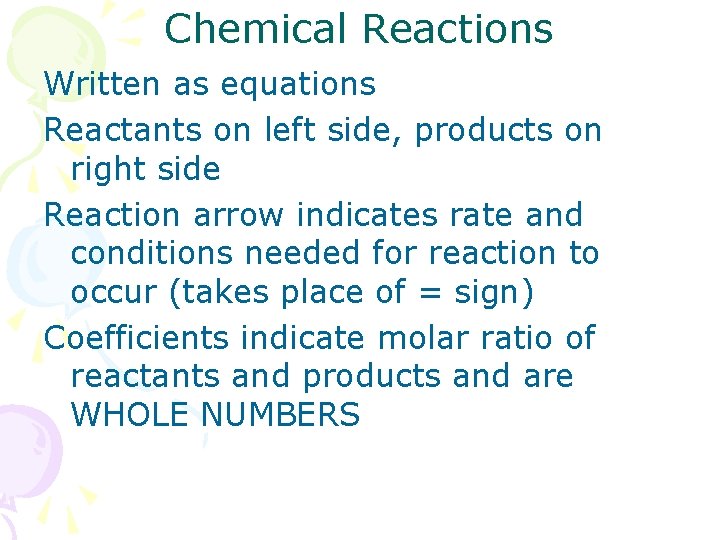
Chemical Reactions Written as equations Reactants on left side, products on right side Reaction arrow indicates rate and conditions needed for reaction to occur (takes place of = sign) Coefficients indicate molar ratio of reactants and products and are WHOLE NUMBERS
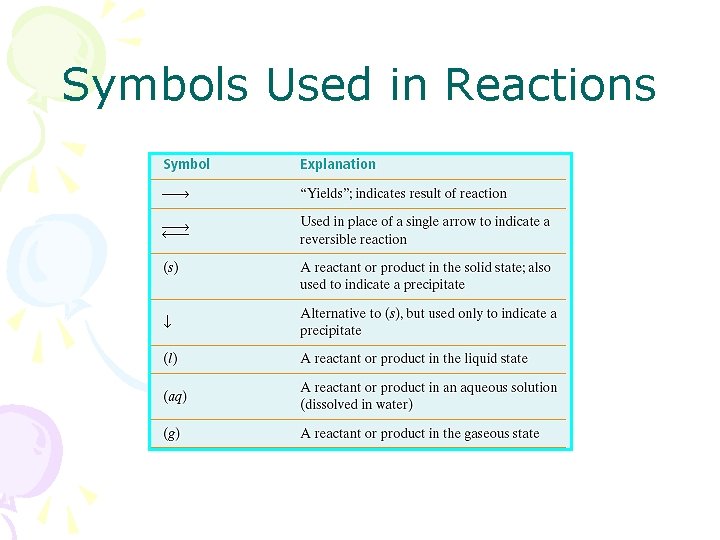
Symbols Used in Reactions
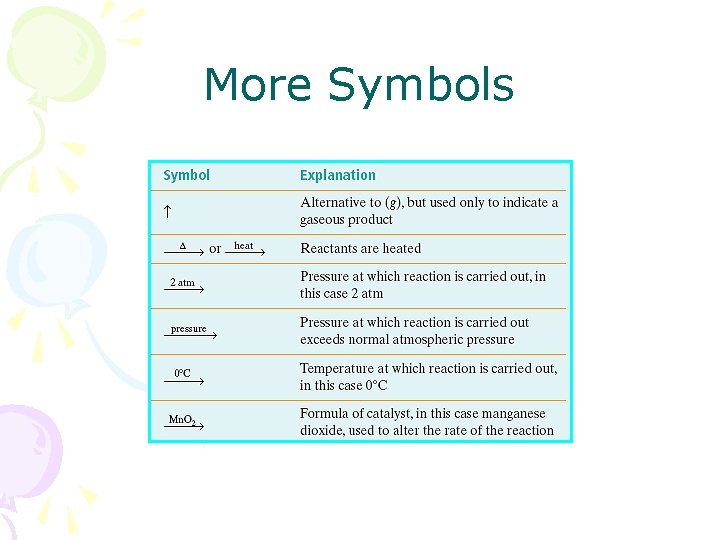
More Symbols
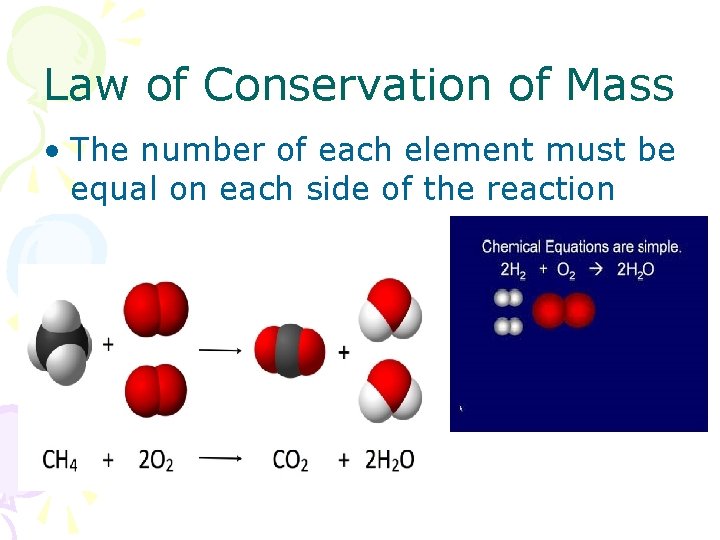
Law of Conservation of Mass • The number of each element must be equal on each side of the reaction
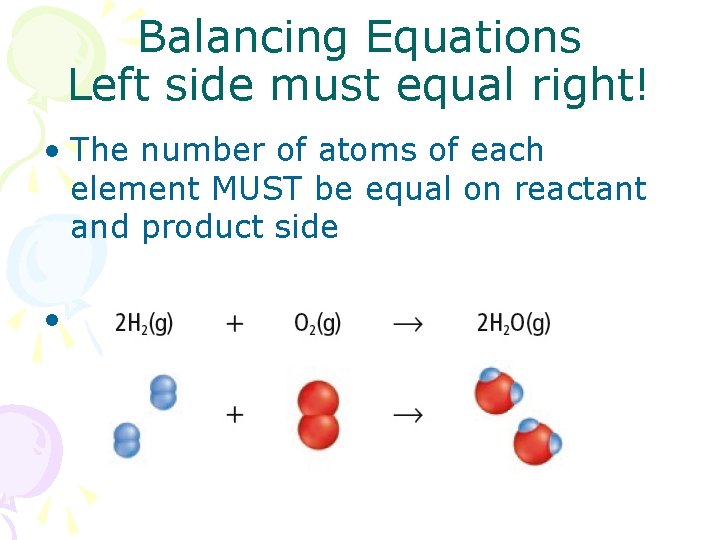
Balancing Equations Left side must equal right! • The number of atoms of each element MUST be equal on reactant and product side •
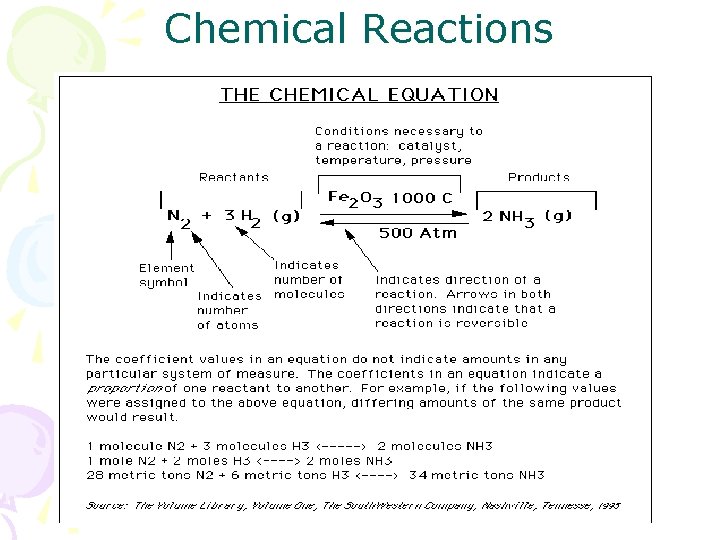
Chemical Reactions
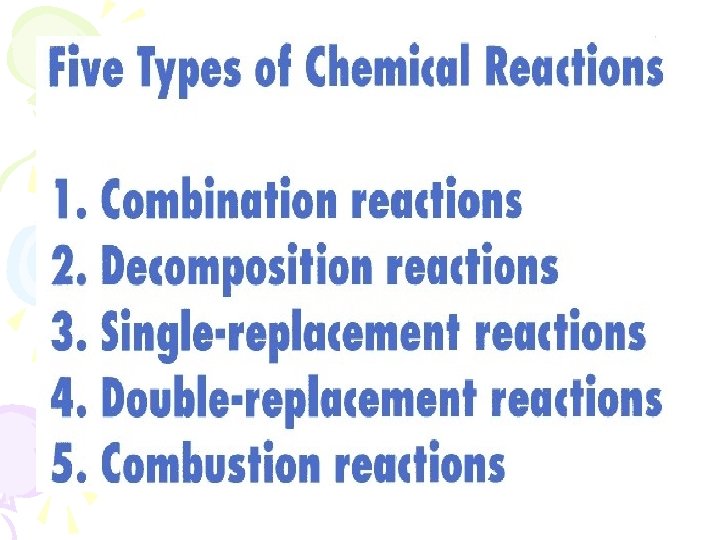
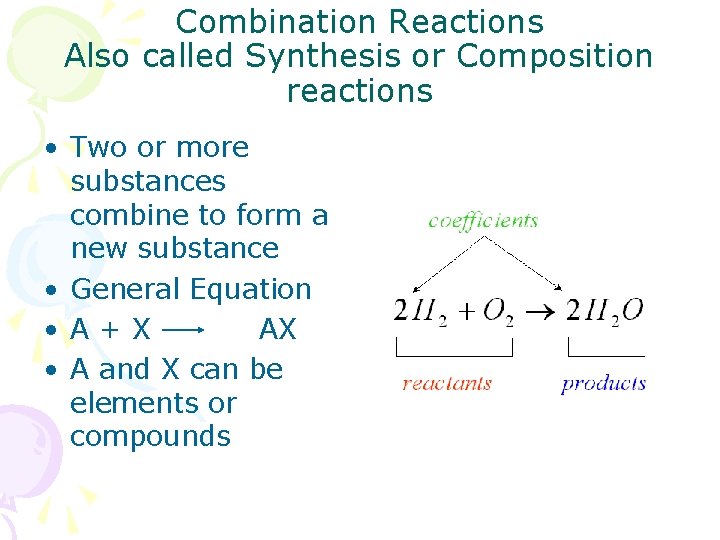
Combination Reactions Also called Synthesis or Composition reactions • Two or more substances combine to form a new substance • General Equation • A+X AX • A and X can be elements or compounds

Common Synthesis Reactions • Elements combine with oxygen to form an oxide ex. Rust is Iron Oxide • Metals react with Halogens to form salts ex. Na. Cl • Active (Group I and II) metal oxides react with water to produce metal hydroxides. Ex. Ca. O quicklime reacts with water to for Calcium hydroxide
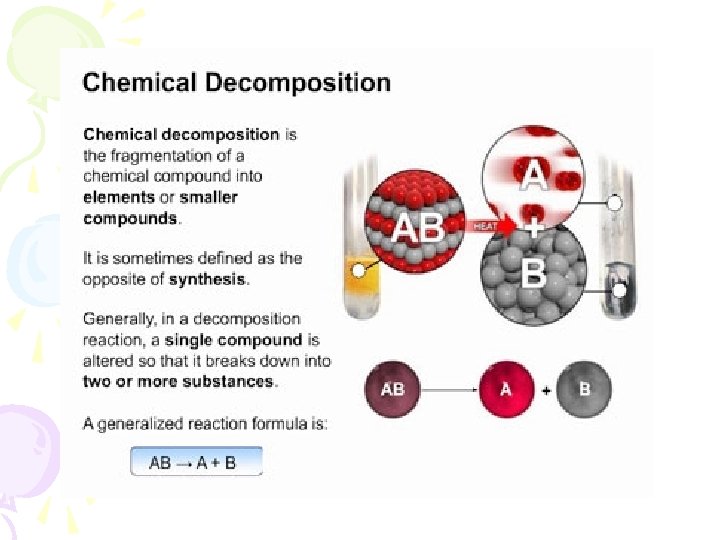
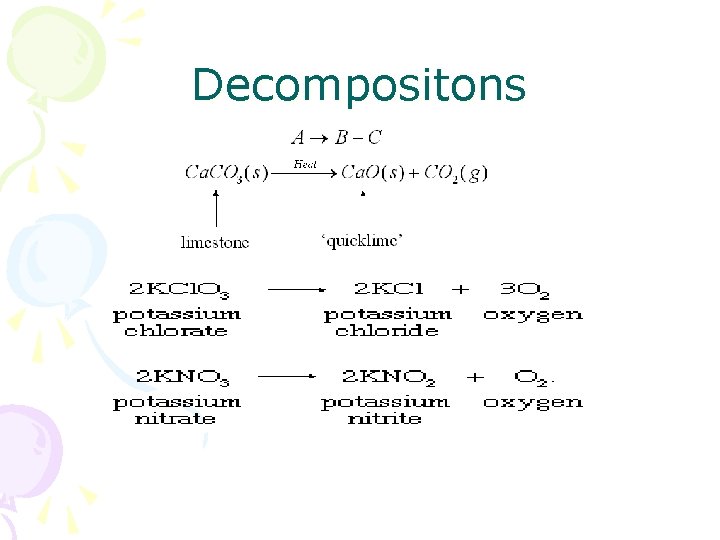
Decompositons
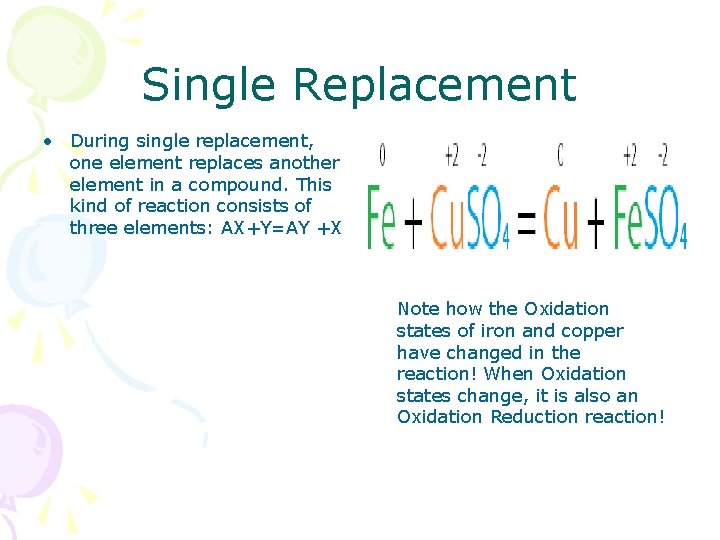
Single Replacement • During single replacement, one element replaces another element in a compound. This kind of reaction consists of three elements: AX+Y=AY +X Note how the Oxidation states of iron and copper have changed in the reaction! When Oxidation states change, it is also an Oxidation Reduction reaction!
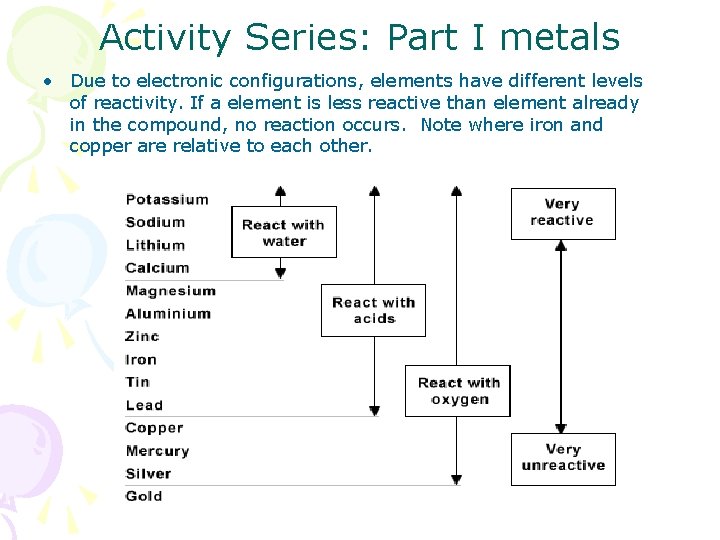
Activity Series: Part I metals • Due to electronic configurations, elements have different levels of reactivity. If a element is less reactive than element already in the compound, no reaction occurs. Note where iron and copper are relative to each other.
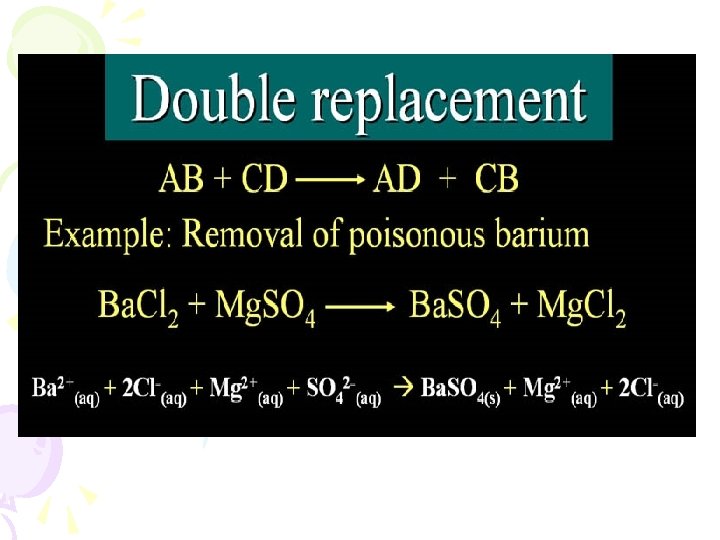
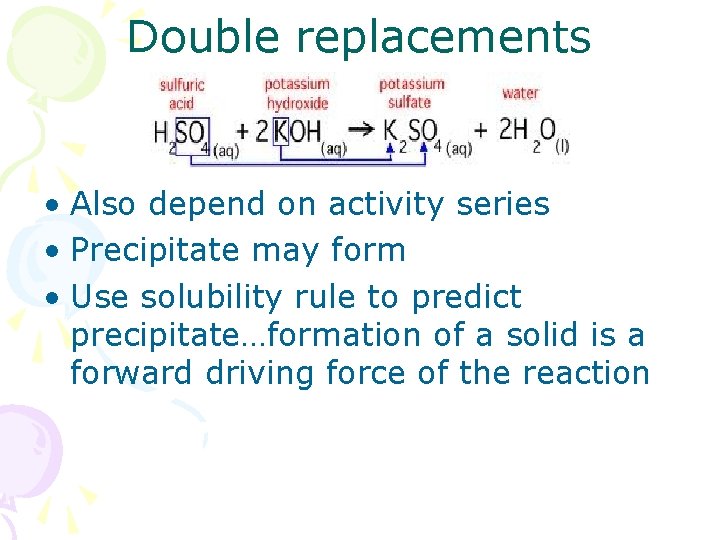
Double replacements • Also depend on activity series • Precipitate may form • Use solubility rule to predict precipitate…formation of a solid is a forward driving force of the reaction

Double Replacements Oxidation states may or may not change. When they do, it is also and Oxidation-Reduction Reaction!
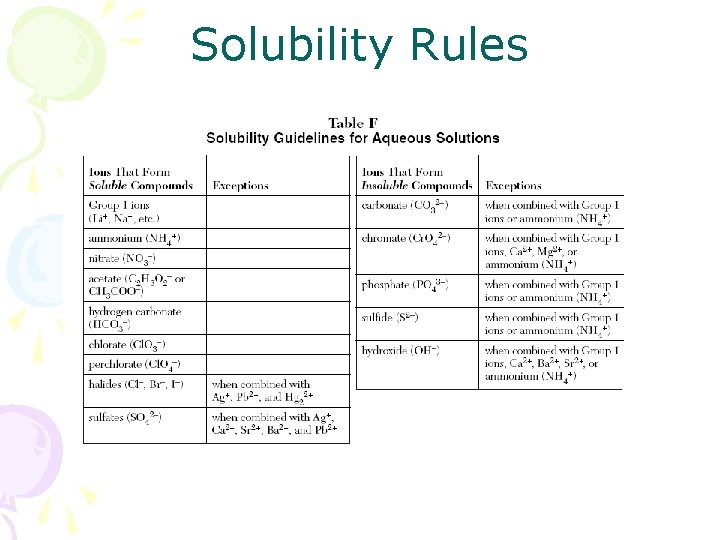
Solubility Rules

Combustion • The combination of a hydrocarbon and oxygen producing water and carbon dioxide • Needs heat (energy input) for reaction to occur • Energy put into reaction causes bond energy in hydrocarbon to be released as heat
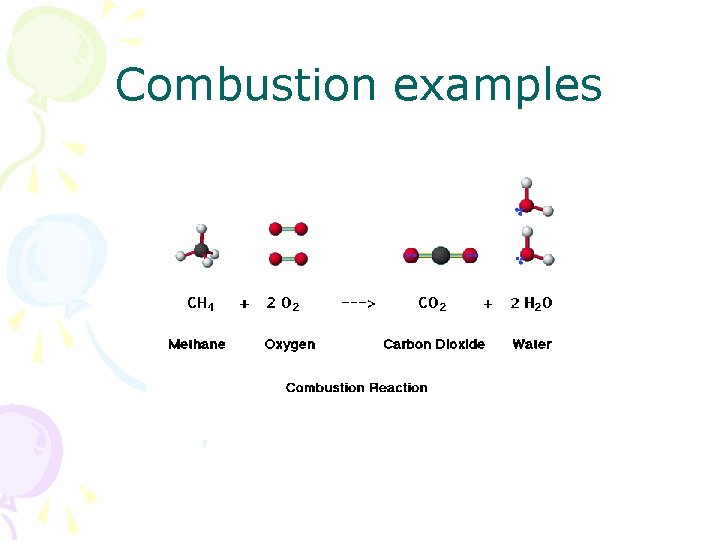
Combustion examples
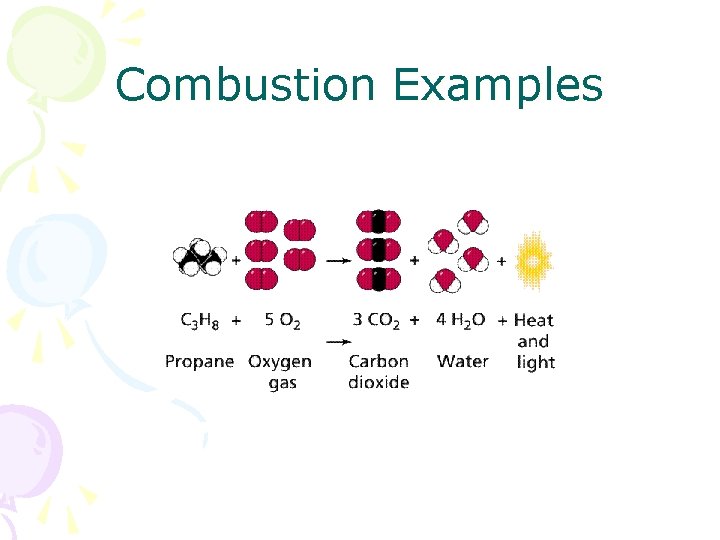
Combustion Examples
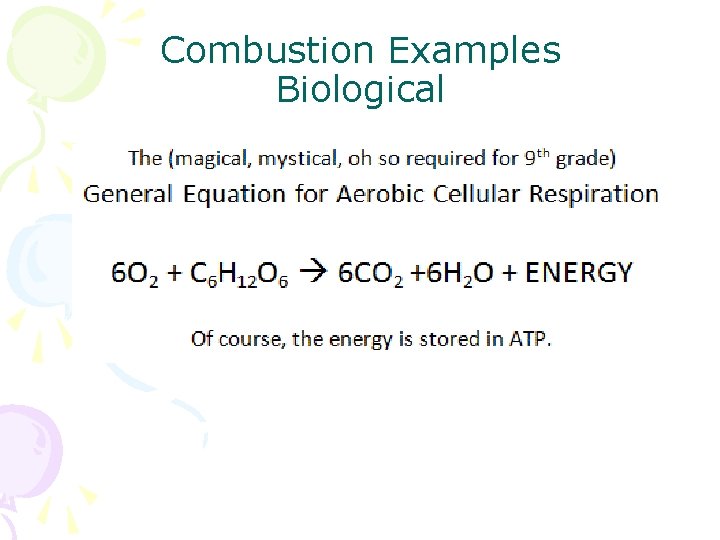
Combustion Examples Biological
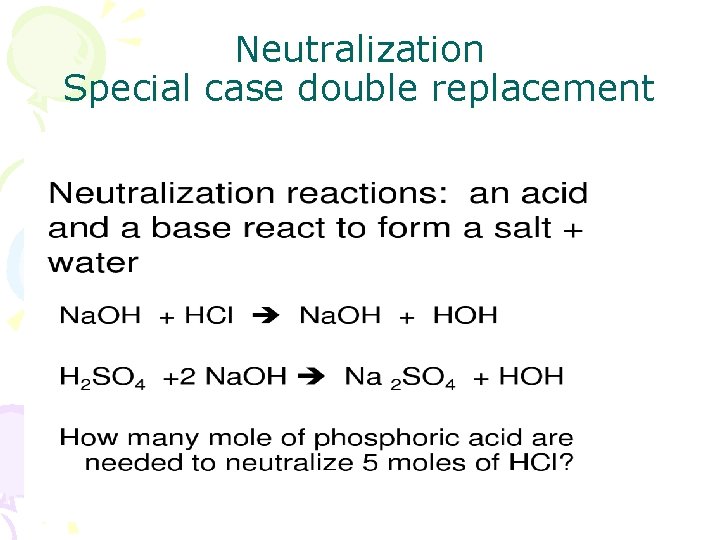
Neutralization Special case double replacement

Common Acids have a hydrogen replaced in neutralization reaction
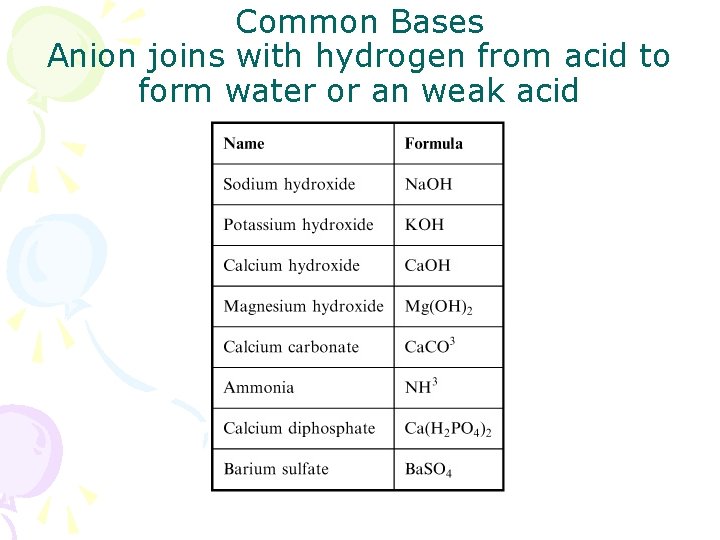
Common Bases Anion joins with hydrogen from acid to form water or an weak acid

Salts • Ionic compounds formed from and acid base reaction • Na. Cl table salt • Mg. SO 2 Epsom salt • Ca. Cl 2 calcium chloride sidewalk salt for colder temperature • Na. I added to table salt to prevent eye diseases “iodized salt”
- Slides: 26