1 Types of Reactions Types of Reactions Chemical
- Slides: 18

1 Types of Reactions

Types of Reactions: Chemical reactions can be classified as: 1) Synthesis or combination reactions 2) Decomposition reactions 3) Single replacement reactions 4) Double replacement reactions 5) Combustion reactions 2

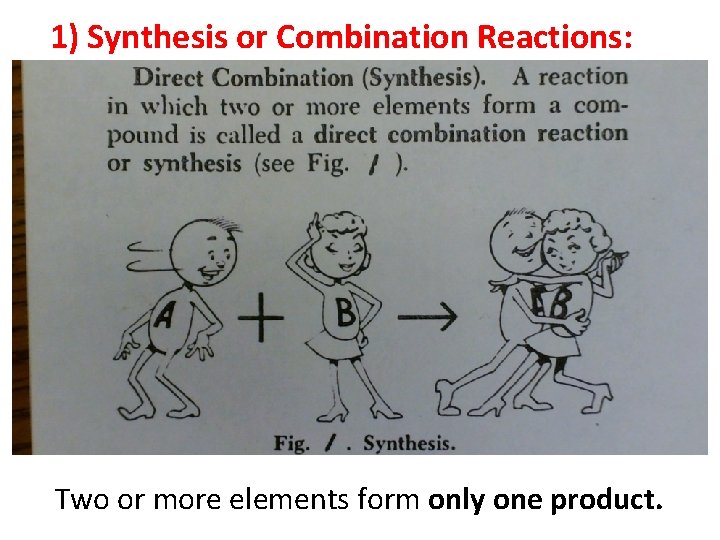
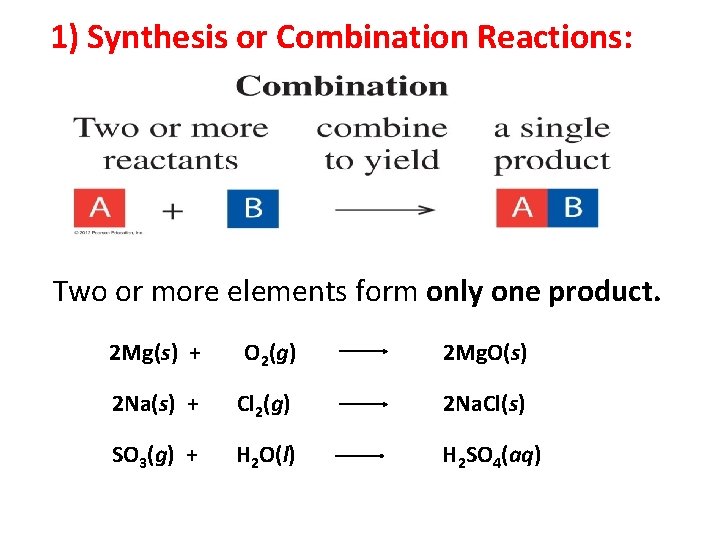
1) Synthesis or Combination Reactions: Two or more elements form only one product. 3

1) Synthesis or Combination Reactions: Two or more elements form only one product. 2 Mg(s) + O 2(g) 2 Mg. O(s) 2 Na(s) + Cl 2(g) 2 Na. Cl(s) SO 3(g) + H 2 O(l) H 2 SO 4(aq) 4

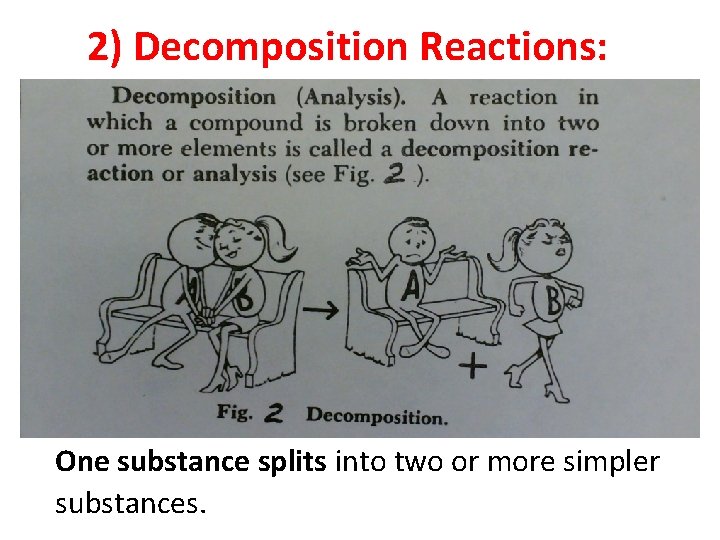
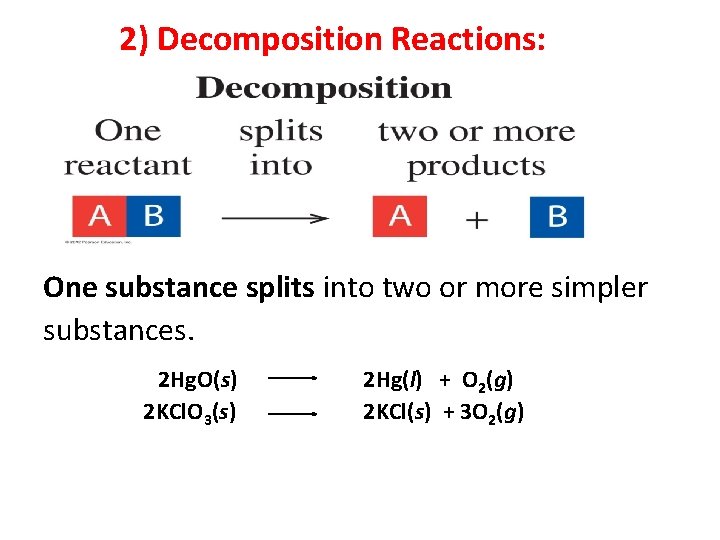
2) Decomposition Reactions: One substance splits into two or more simpler substances.

2) Decomposition Reactions: One substance splits into two or more simpler substances. 2 Hg. O(s) 2 KCl. O 3(s) 2 Hg(l) + O 2(g) 2 KCl(s) + 3 O 2(g) 6

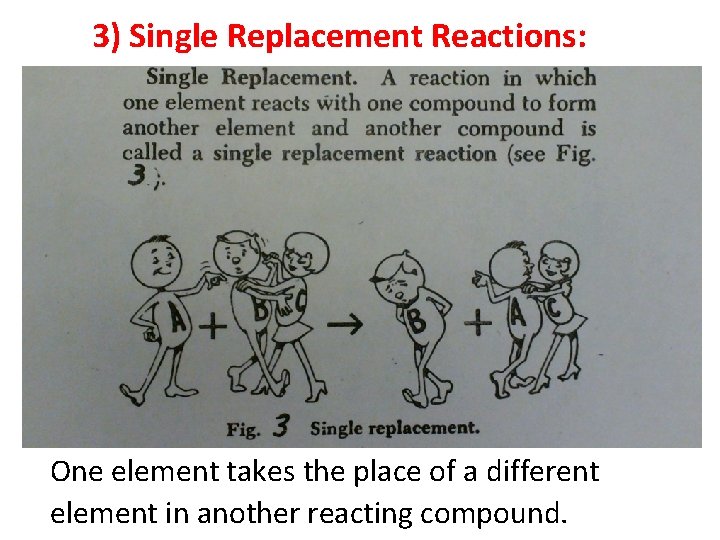
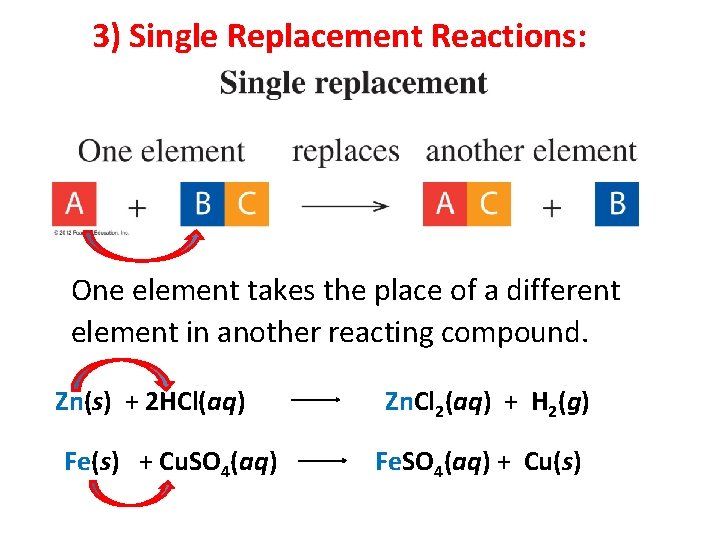
3) Single Replacement Reactions: One element takes the place of a different element in another reacting compound. 7

3) Single Replacement Reactions: One element takes the place of a different element in another reacting compound. Zn(s) + 2 HCl(aq) Fe(s) + Cu. SO 4(aq) Zn. Cl 2(aq) + H 2(g) Fe. SO 4(aq) + Cu(s) 8

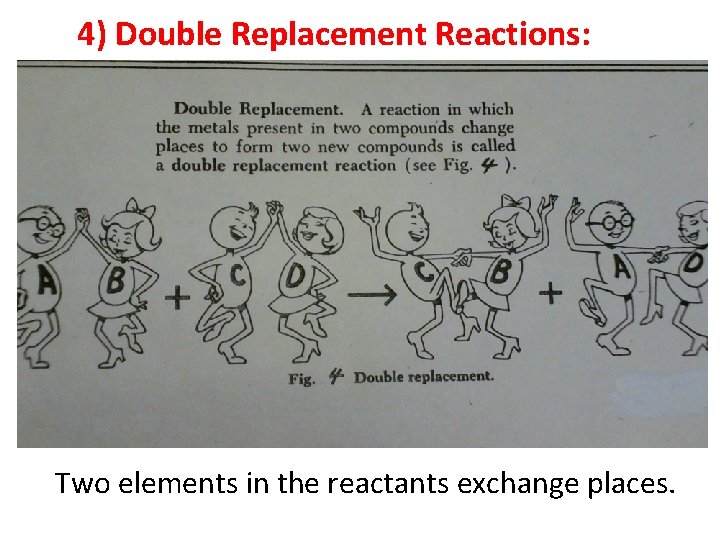
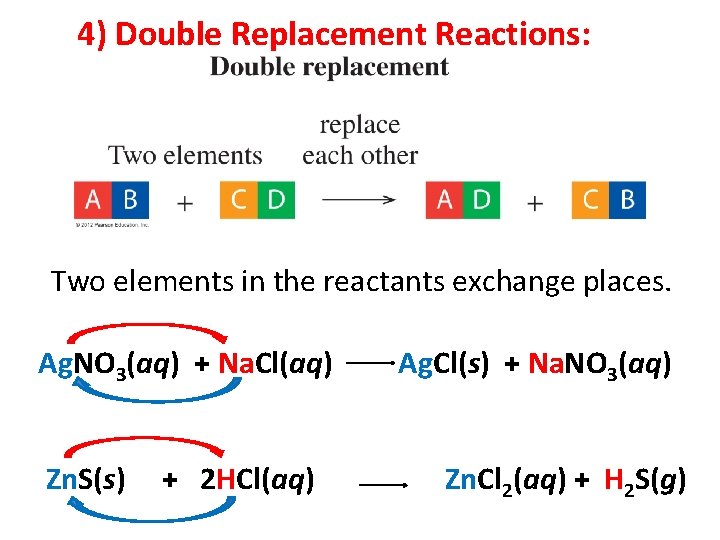
4) Double Replacement Reactions: Two elements in the reactants exchange places.

4) Double Replacement Reactions: Two elements in the reactants exchange places. Ag. NO 3(aq) + Na. Cl(aq) Zn. S(s) + 2 HCl(aq) Ag. Cl(s) + Na. NO 3(aq) Zn. Cl 2(aq) + H 2 S(g)

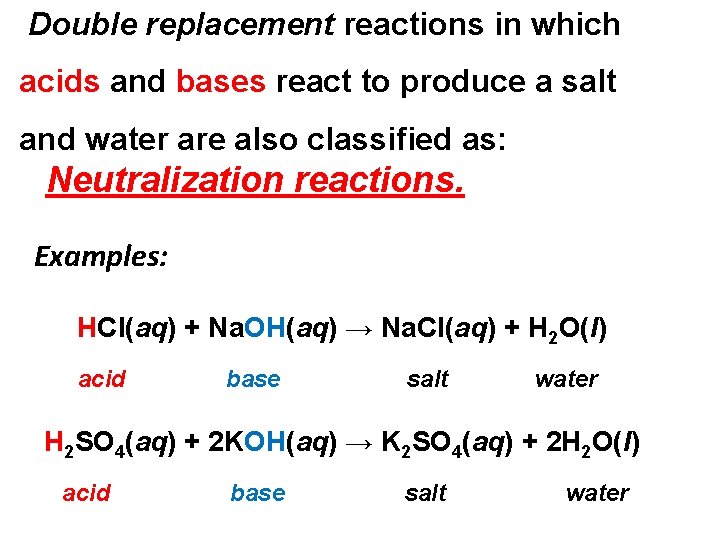
Double replacement reactions in which acids and bases react to produce a salt and water are also classified as: Neutralization reactions. Examples: HCl(aq) + Na. OH(aq) → Na. Cl(aq) + H 2 O(l) acid base salt water H 2 SO 4(aq) + 2 KOH(aq) → K 2 SO 4(aq) + 2 H 2 O(l) acid base salt water

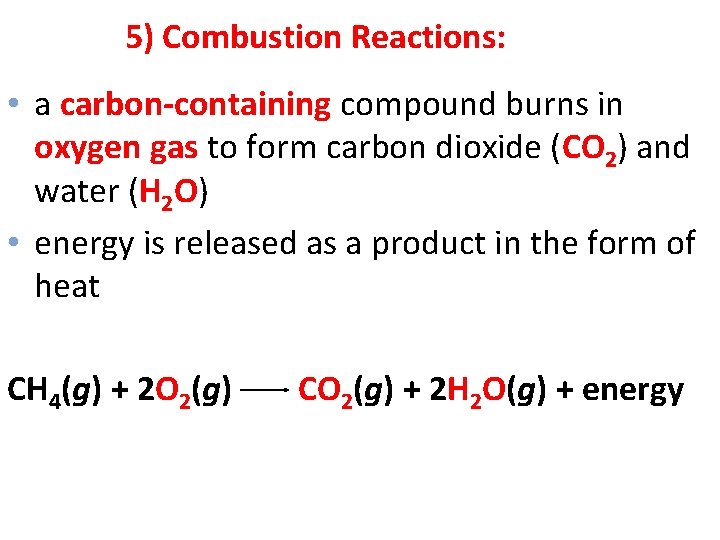
5) Combustion Reactions: • a carbon-containing compound burns in oxygen gas to form carbon dioxide (CO 2) and water (H 2 O) • energy is released as a product in the form of heat CH 4(g) + 2 O 2(g) CO 2(g) + 2 H 2 O(g) + energy 12

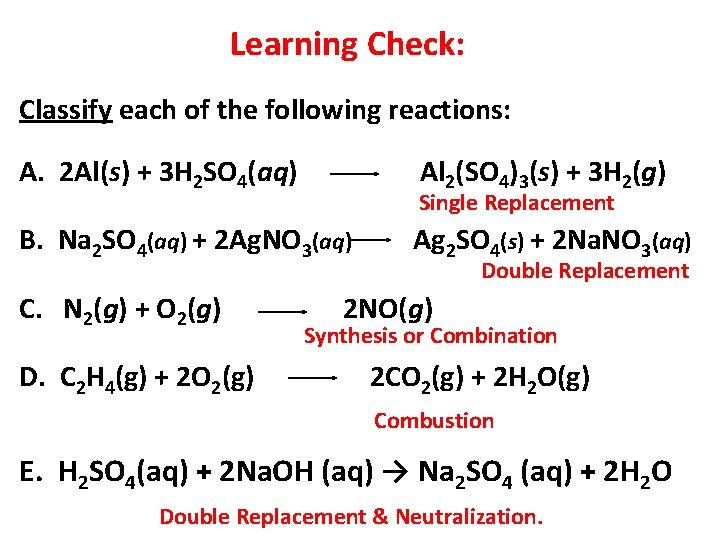
Learning Check: Classify each of the following reactions: A. 2 Al(s) + 3 H 2 SO 4(aq) Al 2(SO 4)3(s) + 3 H 2(g) Single Replacement B. Na 2 SO 4(aq) + 2 Ag. NO 3(aq) C. N 2(g) + O 2(g) D. C 2 H 4(g) + 2 O 2(g) Ag 2 SO 4(s) + 2 Na. NO 3(aq) Double Replacement 2 NO(g) Synthesis or Combination 2 CO 2(g) + 2 H 2 O(g) Combustion E. H 2 SO 4(aq) + 2 Na. OH (aq) → Na 2 SO 4 (aq) + 2 H 2 O Double Replacement & Neutralization. 13

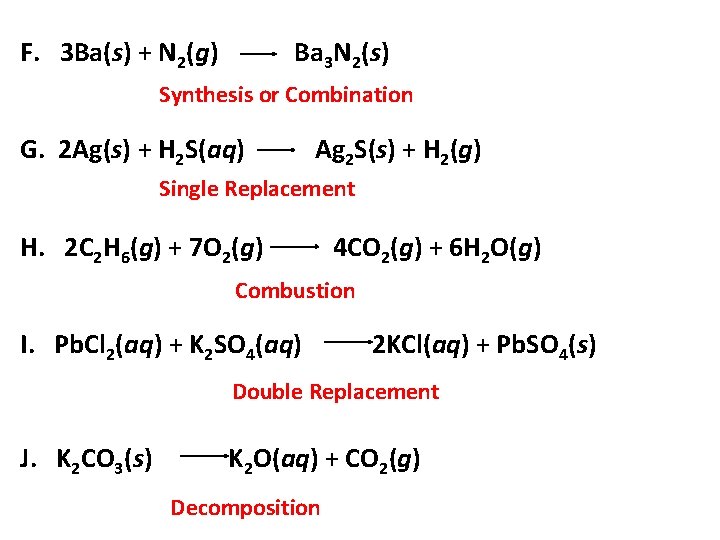
F. 3 Ba(s) + N 2(g) Ba 3 N 2(s) Synthesis or Combination G. 2 Ag(s) + H 2 S(aq) Ag 2 S(s) + H 2(g) Single Replacement H. 2 C 2 H 6(g) + 7 O 2(g) 4 CO 2(g) + 6 H 2 O(g) Combustion I. Pb. Cl 2(aq) + K 2 SO 4(aq) 2 KCl(aq) + Pb. SO 4(s) Double Replacement J. K 2 CO 3(s) K 2 O(aq) + CO 2(g) Decomposition 14

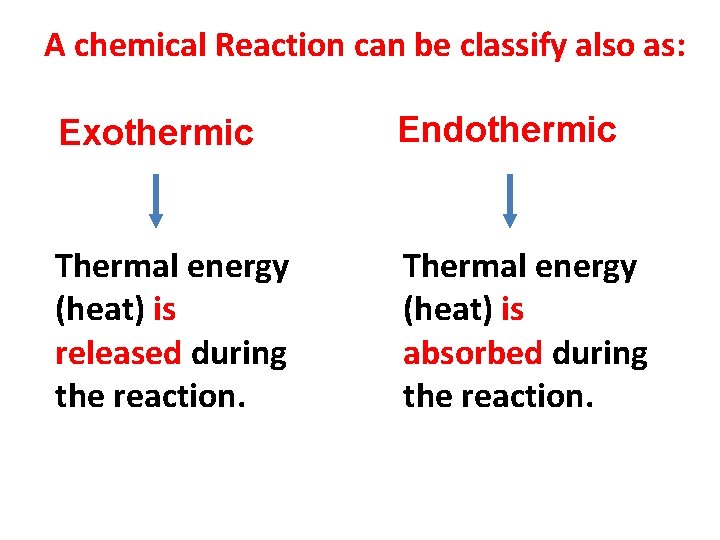
A chemical Reaction can be classify also as: Exothermic Endothermic Thermal energy (heat) is released during the reaction. Thermal energy (heat) is absorbed during the reaction.

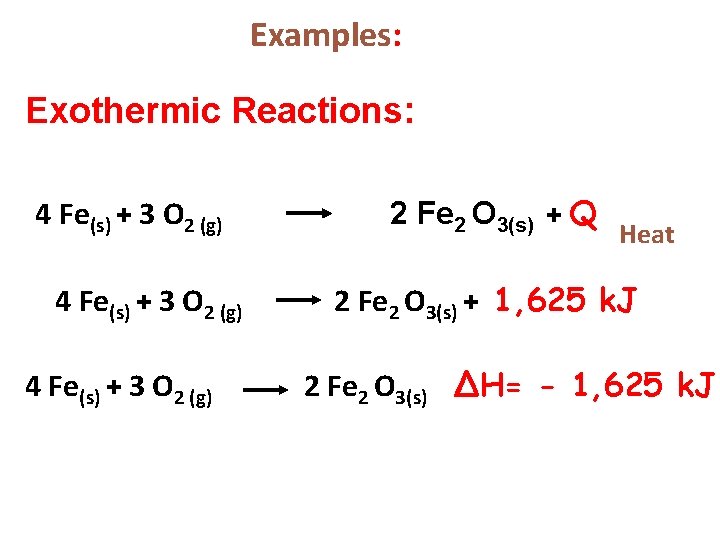
Examples: Exothermic Reactions: 4 Fe(s) + 3 O 2 (g) 2 Fe 2 O 3(s) + Q Heat 2 Fe 2 O 3(s) + 1, 625 k. J 2 Fe 2 O 3(s) ∆H= - 1, 625 k. J

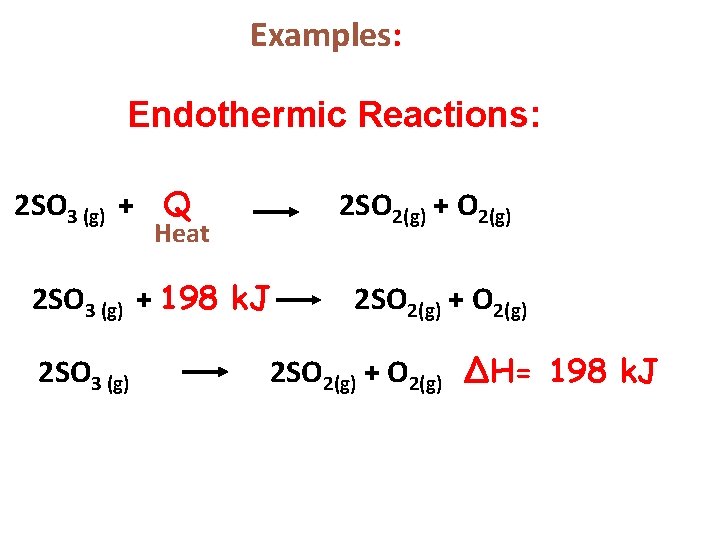
Examples: Endothermic Reactions: 2 SO 3 (g) + Q 2 SO 2(g) + O 2(g) Heat 2 SO 3 (g) + 198 k. J 2 SO 3 (g) 2 SO 2(g) + O 2(g) ∆H= 198 k. J

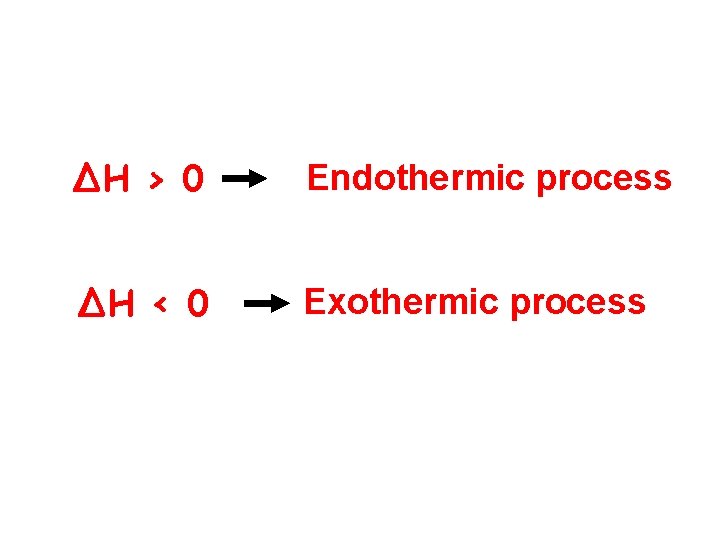
∆H › 0 Endothermic process ∆H ‹ 0 Exothermic process
 Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Types of reactions
Types of reactions Section 1 chemical changes
Section 1 chemical changes Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations Types of redox reactions
Types of redox reactions Types of reactions
Types of reactions Types of reactions chemistry
Types of reactions chemistry Reaction type
Reaction type 4 types of chemical reactions
4 types of chemical reactions Four types of chemical reactions
Four types of chemical reactions Five chemical changes
Five chemical changes 5 general types of chemical reactions
5 general types of chemical reactions What are the 4 types of chemical reactions
What are the 4 types of chemical reactions Four types of chemical reactions
Four types of chemical reactions Types of reactions
Types of reactions Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Three types of chemical reactions
Three types of chemical reactions Combustion reaction
Combustion reaction


































