Types of Chemical Reactions Chemical Reactions A chemical






- Slides: 16

Types of Chemical Reactions

Chemical Reactions �A chemical reaction has occurred when the starting substances (reactants) recombine to form ending substances (products). �The reactants will, most often, have different chemical and physical properties than the products. �Evidence of a chemical reaction can include (but is not limited to): bubbles, flames, change of color, change of state, precipitate.

Basic Types of Chemical Reactions �Synthesis (or combination) �Decomposition �Single Replacement �Double Replacement �Combustion

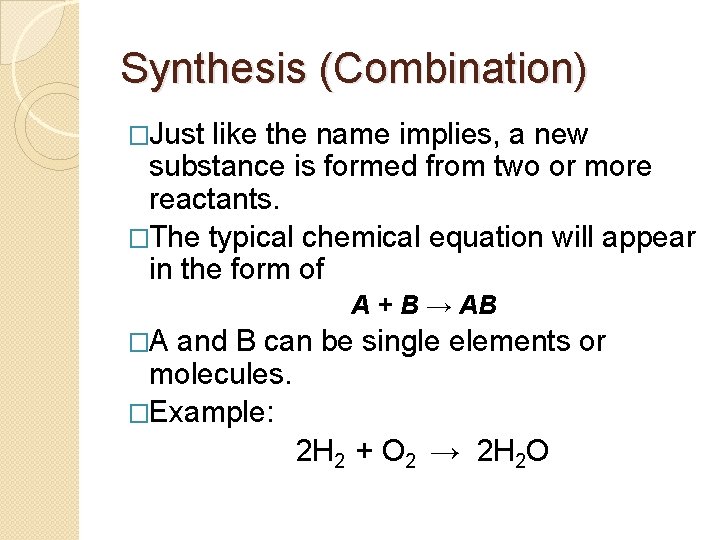
Synthesis (Combination) �Just like the name implies, a new substance is formed from two or more reactants. �The typical chemical equation will appear in the form of A + B → AB �A and B can be single elements or molecules. �Example: 2 H 2 + O 2 → 2 H 2 O

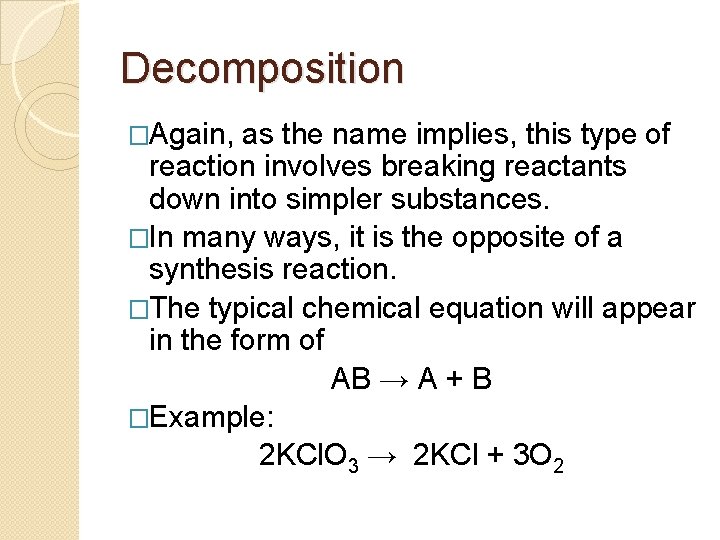
Decomposition �Again, as the name implies, this type of reaction involves breaking reactants down into simpler substances. �In many ways, it is the opposite of a synthesis reaction. �The typical chemical equation will appear in the form of AB → A + B �Example: 2 KCl. O 3 → 2 KCl + 3 O 2

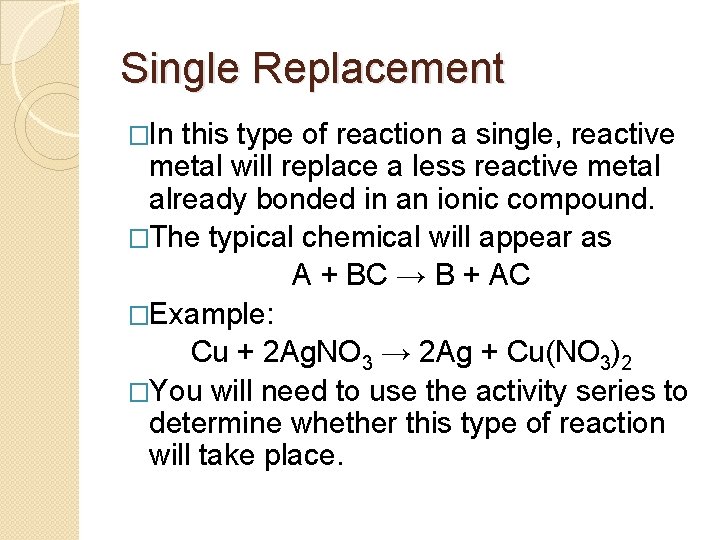
Single Replacement �In this type of reaction a single, reactive metal will replace a less reactive metal already bonded in an ionic compound. �The typical chemical will appear as A + BC → B + AC �Example: Cu + 2 Ag. NO 3 → 2 Ag + Cu(NO 3)2 �You will need to use the activity series to determine whether this type of reaction will take place.

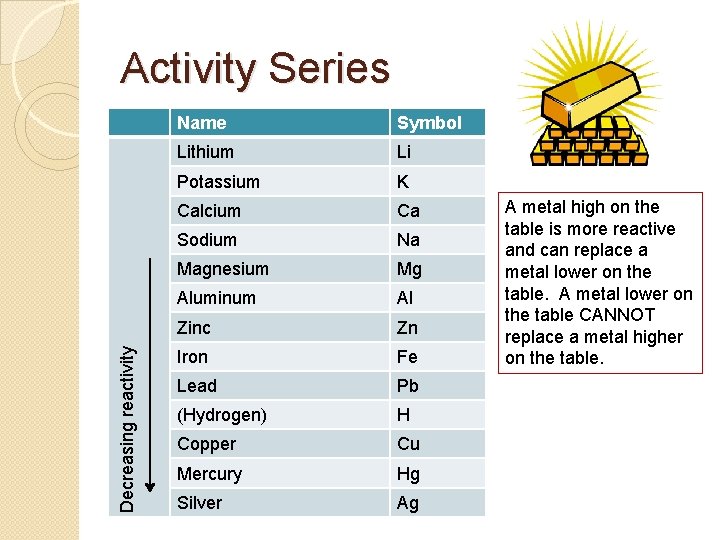
Activity Series �The only time a single replacement will take place is when the free metal is more reactive than the metal cation bonded in the ionic compound. �This reactivity is based on ionization energy. A metal with a lower ionization energy is more reactive than one with a higher ionization energy. �You will need to use the activity series to determine which metals are more reactive than others.

Decreasing reactivity Activity Series Name Symbol Lithium Li Potassium K Calcium Ca Sodium Na Magnesium Mg Aluminum Al Zinc Zn Iron Fe Lead Pb (Hydrogen) H Copper Cu Mercury Hg Silver Ag A metal high on the table is more reactive and can replace a metal lower on the table. A metal lower on the table CANNOT replace a metal higher on the table.

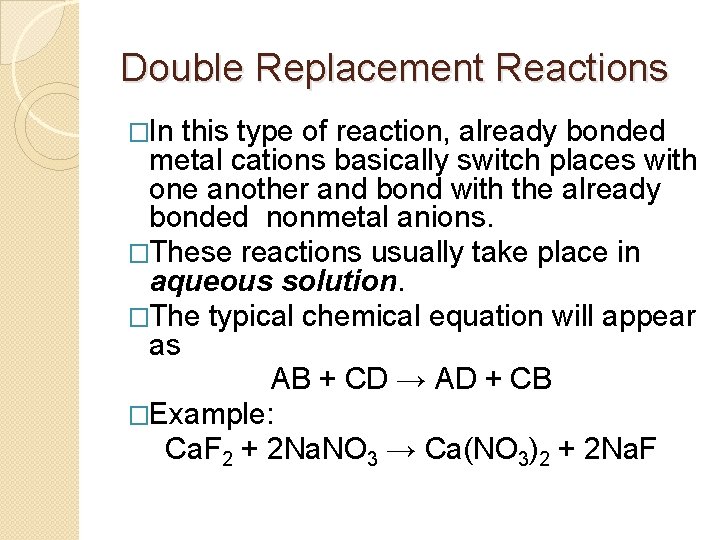
Double Replacement Reactions �In this type of reaction, already bonded metal cations basically switch places with one another and bond with the already bonded nonmetal anions. �These reactions usually take place in aqueous solution. �The typical chemical equation will appear as AB + CD → AD + CB �Example: Ca. F 2 + 2 Na. NO 3 → Ca(NO 3)2 + 2 Na. F

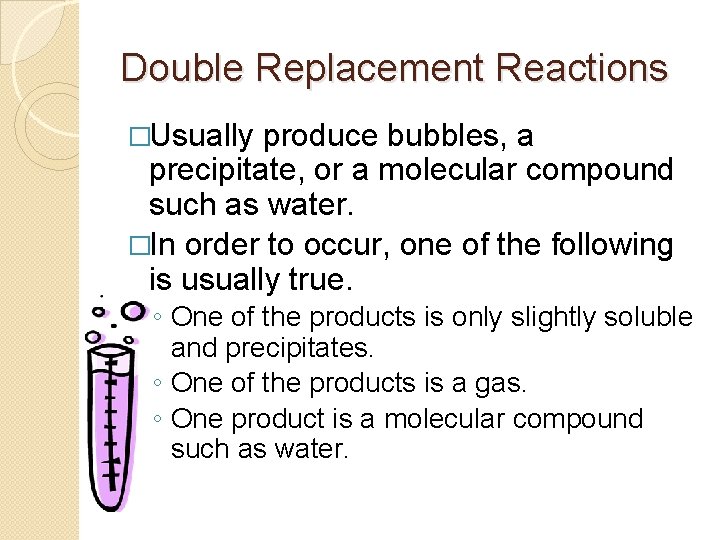
Double Replacement Reactions �Usually produce bubbles, a precipitate, or a molecular compound such as water. �In order to occur, one of the following is usually true. ◦ One of the products is only slightly soluble and precipitates. ◦ One of the products is a gas. ◦ One product is a molecular compound such as water.

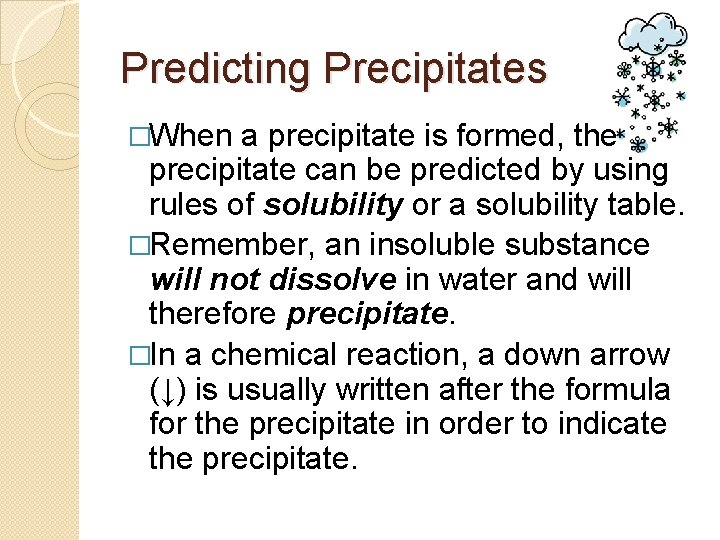
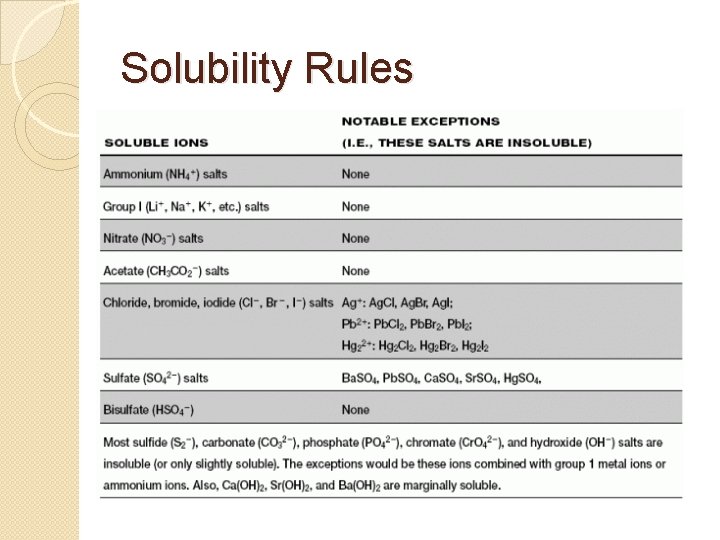
Predicting Precipitates �When a precipitate is formed, the precipitate can be predicted by using rules of solubility or a solubility table. �Remember, an insoluble substance will not dissolve in water and will therefore precipitate. �In a chemical reaction, a down arrow (↓) is usually written after the formula for the precipitate in order to indicate the precipitate.

Solubility Rules

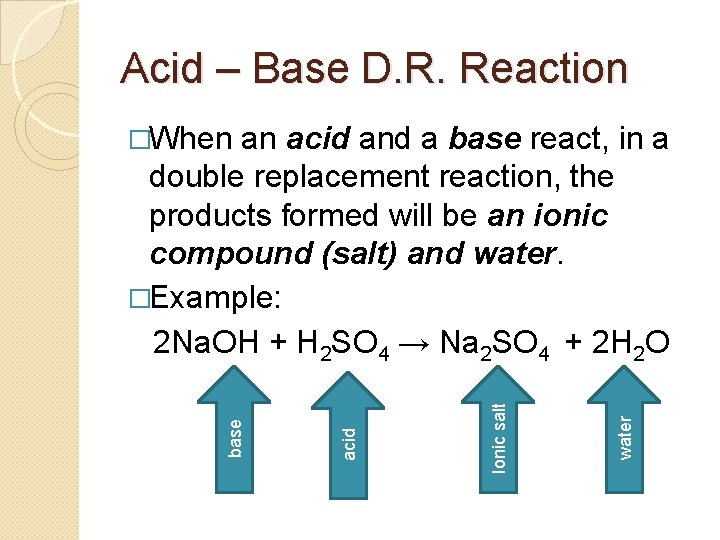
Acid – Base Double Replacement �An acid, for now, is defined as a substance that produces a hydrogen ion in solution. We will recognize an acid as an ionic compound that starts with H. �Example: HCl, H 2 SO 4 �A base, for now, is defined as a substance that produces a hydroxide ion in solution. We will recognize a base as an ionic compound that ends with – OH. �Example: Na. OH, Ca(OH)2

Acid – Base D. R. Reaction water Ionic salt acid and a base react, in a double replacement reaction, the products formed will be an ionic compound (salt) and water. �Example: 2 Na. OH + H 2 SO 4 → Na 2 SO 4 + 2 H 2 O base �When

Combustion Reaction �In a combustion reaction, a hydrocarbon burns in oxygen. The products formed are always carbon dioxide and water. �The typical format for this reaction is Cx. Hy + O 2 → CO 2 + H 2 O �Example: CH 4 + 2 O 2 → CO 2 + 2 H 2 O

Try These Worksheets! � http: //misterguch. brinkster. net/6 typesofreaction. pdf http: //www. everettcc. edu/uploaded. Files/Student_Resources_ and_Services/TRIO/Types_of_reactions_worksheet. pdf � http: //www. sciencegeek. net/Chemistry/chempdfs/Equations. W orksheet 4. pdf � http: //www. sciencegeek. net/Chemistry/chempdfs/Equations. W orksheet 2. pdf � http: //www. sciencegeek. net/Chemistry/chempdfs/Equations. W orksheet 3. pdf � http: //misterguch. brinkster. net/equationworksheets. html � ◦ From this link, choose reaction products worksheet, six types of reactions, or double displacement reactions
 Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Section 1 chemical changes
Section 1 chemical changes Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations Types of redox reactions
Types of redox reactions Types of reactions
Types of reactions 4 types of chemical reactions
4 types of chemical reactions 5 types of chemical reactions
5 types of chemical reactions 4 types of chemical reactions
4 types of chemical reactions Four types of chemical reactions
Four types of chemical reactions What are five chemical changes
What are five chemical changes 5 general types of chemical reactions
5 general types of chemical reactions What are the 4 types of chemical reactions
What are the 4 types of chemical reactions Four types of chemical reactions
Four types of chemical reactions Chapter 11 chemical reactions answers
Chapter 11 chemical reactions answers Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry






























