Chemical Reactions Types of Chemical Reactions 5 common





- Slides: 24

Chemical Reactions

Types of Chemical Reactions 5 common types of chemical reactions 1. Synthesis 2. Decomposition 3. Single replacement 4. Double replacement 5. Combustion


The 5 Signs of a Chemical Reaction There are five main signs that a chemical reaction has taken place: Light released Temperature change Production of a gas Formation of a precipitate Color change

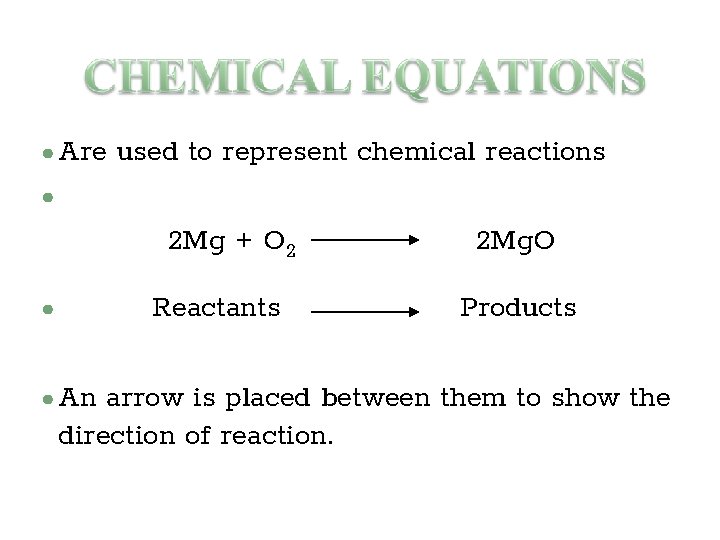
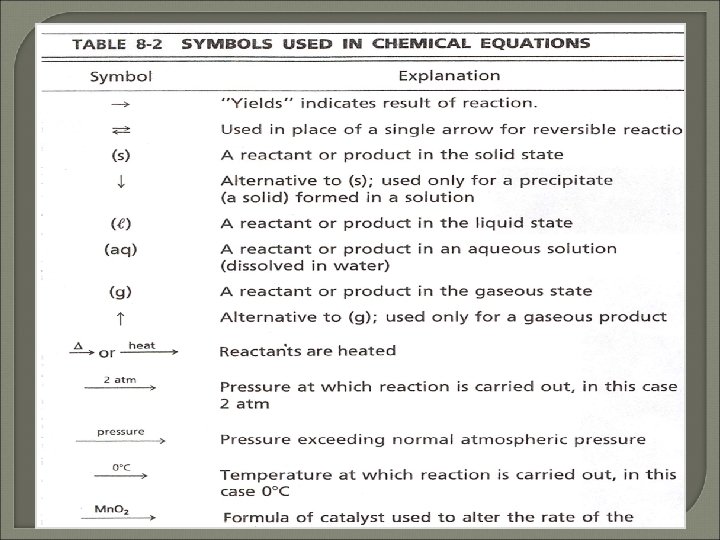
● Are used to represent chemical reactions ● 2 Mg + O 2 Reactants ● ● An 2 Mg. O Products arrow is placed between them to show the direction of reaction.

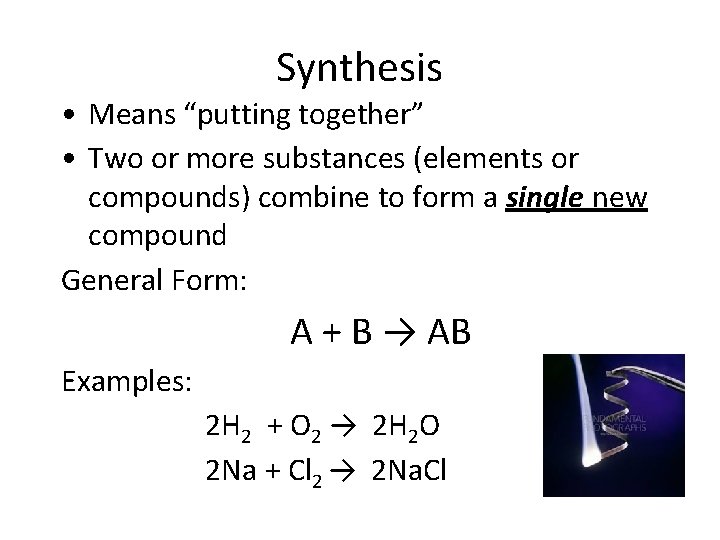
Synthesis • Means “putting together” • Two or more substances (elements or compounds) combine to form a single new compound General Form: A + B → AB Examples: 2 H 2 + O 2 → 2 H 2 O 2 Na + Cl 2 → 2 Na. Cl


Decomposition • Opposite of synthesis reactions • a single compound reacts to give two or more products General Form: AB → A + B Examples: 2 H 2 O → 2 H 2 + O 2 2 Na. Cl → 2 Na + Cl 2

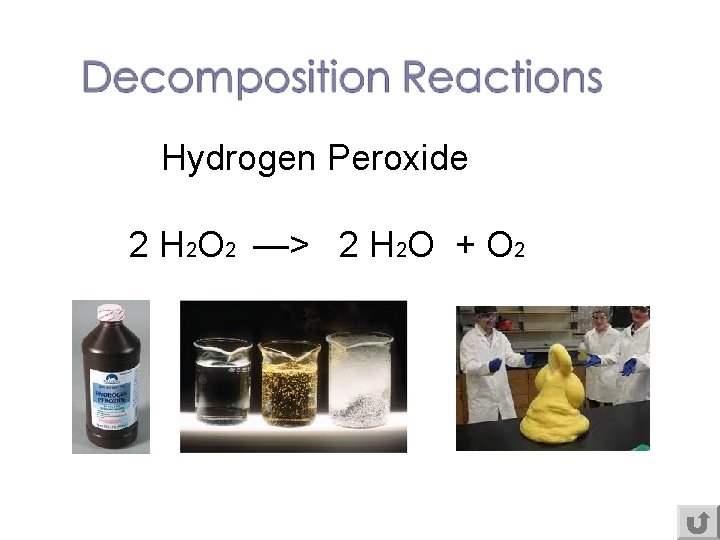
Hydrogen Peroxide 2 H 2 O 2 —> 2 H 2 O + O 2


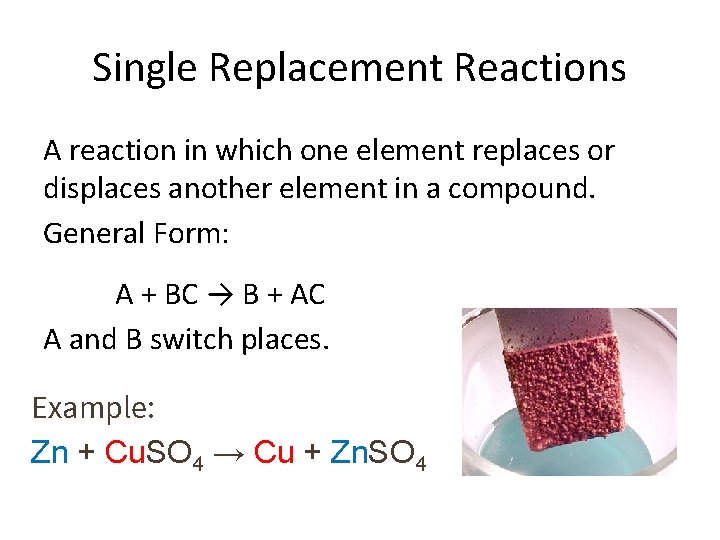
Single Replacement Reactions A reaction in which one element replaces or displaces another element in a compound. General Form: A + BC → B + AC A and B switch places. Example: Zn + Cu. SO 4 → Cu + Zn. SO 4

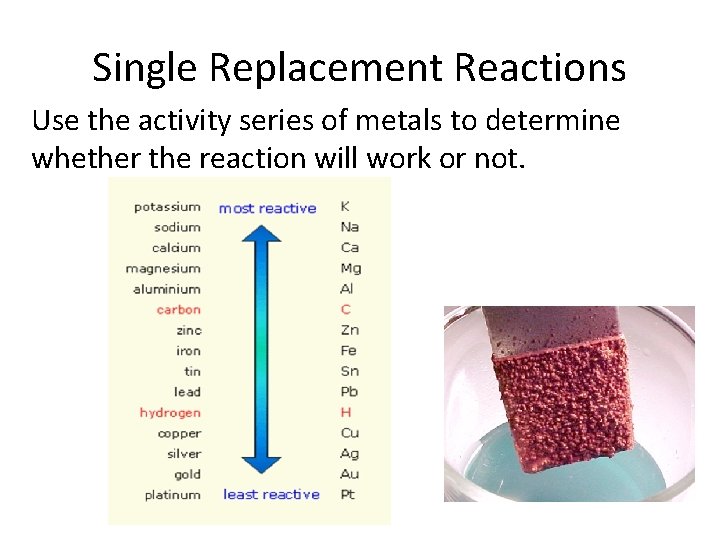
Single Replacement Reactions Use the activity series of metals to determine whether the reaction will work or not.

Double Replacement Reactions A reaction in which two soluble ionic compounds react to form a precipitate (or water). General Form: AC + BD→ AD + BC A and B switch places. Example: Ag. NO 3 + Na. Cl → Na. NO 3 + Ag. Cl (ppt)

Double Replacement Reactions Ag. NO 3(aq) + KCl(aq) ‑‑‑‑> Ag. Cl(s) + KNO 3(aq) Use a solubility table to determine if a precipitate is formed.


Combustion Reactions The burning of a hydrocarbon in the presence of oxygen. Hydrocarbon: organic compound that is composed only of carbon and hydrogen. Examples: gasoline, kerosene, propane

Combustion Reactions General Form: Cx. Hy + O 2 → CO 2 + H 2 O + heat The products are usually carbon dioxide, water, and energy in the form of heat.

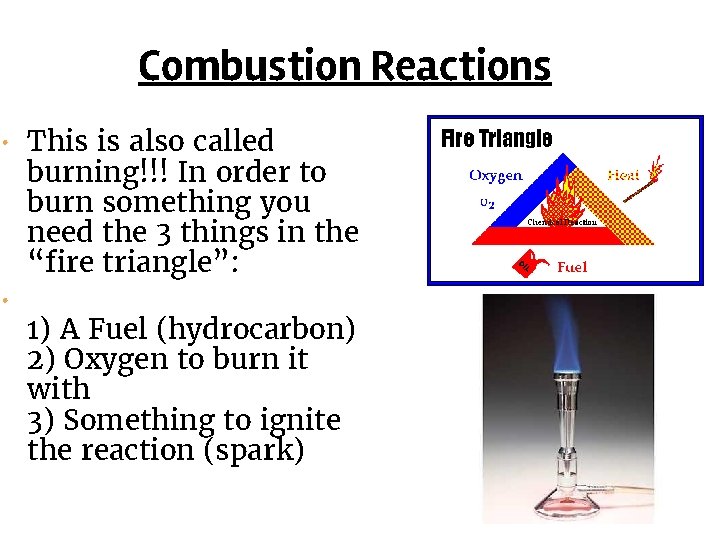
Combustion Reactions • This is also called • burning!!! In order to burn something you need the 3 things in the “fire triangle”: 1) A Fuel (hydrocarbon) 2) Oxygen to burn it with 3) Something to ignite the reaction (spark)

Combustion Reactions • In general: Cx. Hy + O 2 → CO 2 + H 2 O • Products in combustion are ALWAYS carbon dioxide and water. • Combustion is used to heat homes and run automobiles (gasoline is octane, C 8 H 18) • (Note: incomplete burning causes some by-products like carbon monoxide = CO)

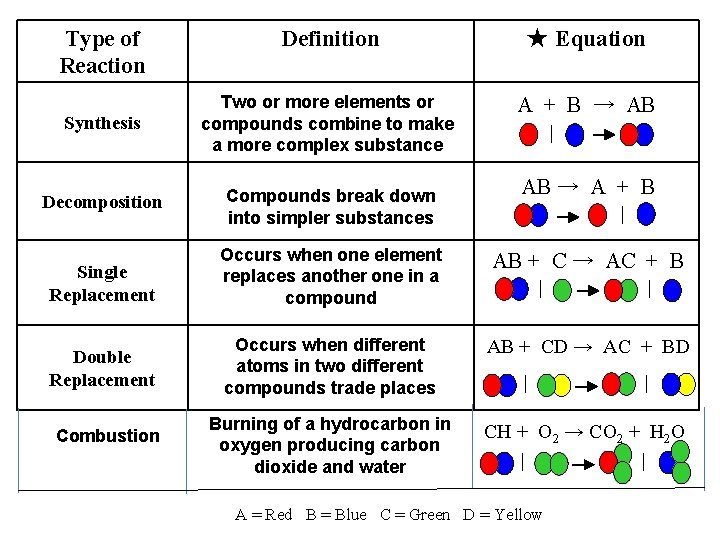
Type of Reaction Definition ★ Equation Synthesis Two or more elements or compounds combine to make a more complex substance A + B → AB Decomposition Compounds break down into simpler substances Single Replacement Occurs when one element replaces another one in a compound AB + C → AC + B Double Replacement Occurs when different atoms in two different compounds trade places AB + CD → AC + BD Burning of a hydrocarbon in oxygen producing carbon dioxide and water CH + O 2 → CO 2 + H 2 O Combustion AB → A + B A = Red B = Blue C = Green D = Yellow

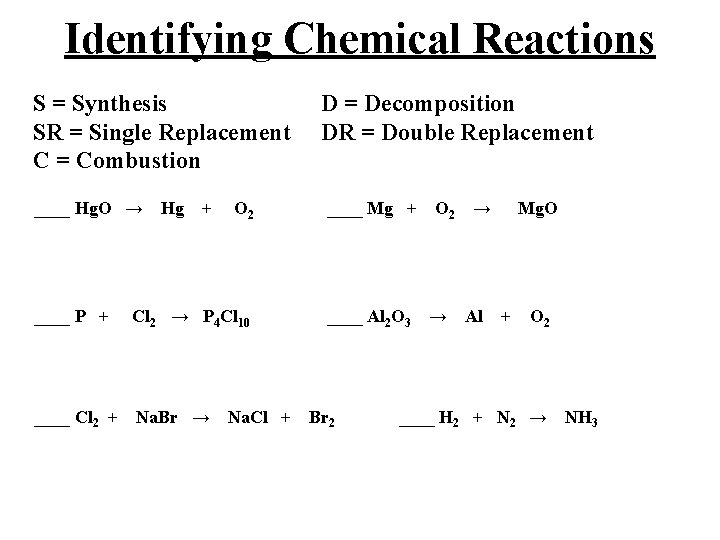
Identifying Chemical Reactions S = Synthesis D = Decomposition SR = Single Replacement DR = Double Replacement C = Combustion ____ Hg. O → Hg + O 2 ____ Mg + O 2 → Mg. O ____ P + Cl 2 → P 4 Cl 10 ____ Al 2 O 3 → Al + O 2 ____ Cl 2 + Na. Br → Na. Cl + Br 2 ____ H 2 + N 2 → NH 3

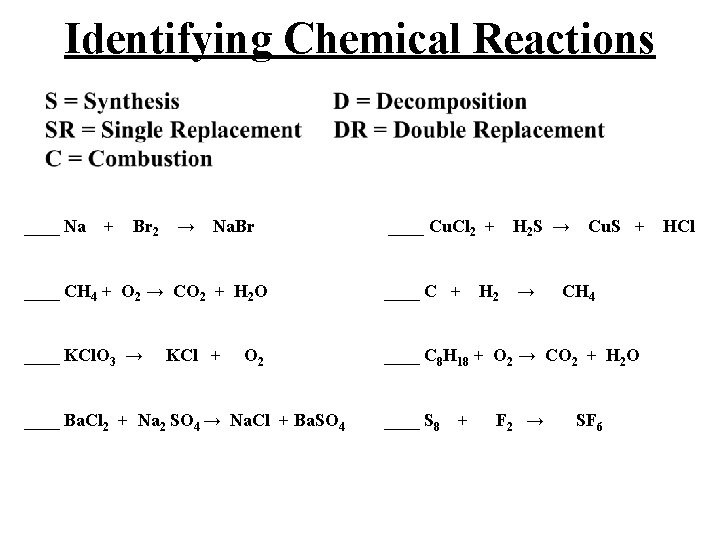
Identifying Chemical Reactions ____ Na + Br 2 → Na. Br ____ CH 4 + O 2 → CO 2 + H 2 O ____ Cu. Cl 2 + H 2 S → Cu. S + HCl ____ C + H 2 → CH 4 ____ KCl. O 3 → KCl + O 2 ____ C 8 H 18 + O 2 → CO 2 + H 2 O ____ Ba. Cl 2 + Na 2 SO 4 → Na. Cl + Ba. SO 4 ____ S 8 + F 2 → SF 6

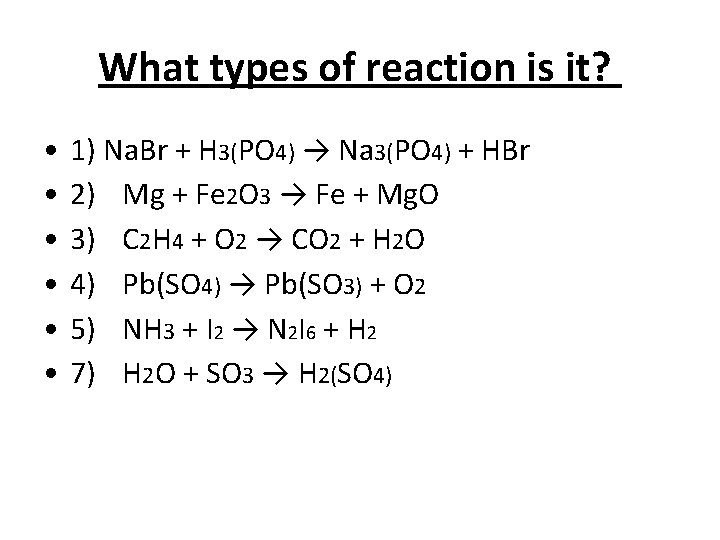
What types of reaction is it? • • • 1) Na. Br + H 3(PO 4) → Na 3(PO 4) + HBr 2) Mg + Fe 2 O 3 → Fe + Mg. O 3) C 2 H 4 + O 2 → CO 2 + H 2 O 4) Pb(SO 4) → Pb(SO 3) + O 2 5) NH 3 + I 2 → N 2 I 6 + H 2 7) H 2 O + SO 3 → H 2(SO 4)

 Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Types of reactions
Types of reactions Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Are kc and kp equal
Are kc and kp equal Different types of redox reactions
Different types of redox reactions How to identify types of chemical reactions
How to identify types of chemical reactions Types of reactions chemistry
Types of reactions chemistry Reaction type
Reaction type 4 types of chemical reactions
4 types of chemical reactions Four types of chemical reactions
Four types of chemical reactions Five chemical
Five chemical 5 general types of chemical reactions
5 general types of chemical reactions What are the 4 types of chemical reactions
What are the 4 types of chemical reactions Four types of chemical reactions
Four types of chemical reactions Chapter 11 chemical reactions answers
Chapter 11 chemical reactions answers Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Fatoumata dembele chef
Fatoumata dembele chef Combustion reaction
Combustion reaction Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Redox half reactions
Redox half reactions Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Proportional relationships in chemical reactions
Proportional relationships in chemical reactions Unit 5 chemical equations and reactions
Unit 5 chemical equations and reactions Predicting products of chemical reactions
Predicting products of chemical reactions














































