Types of Reactions Unit 7 Chemical Reactions Ch

- Slides: 9

Types of Reactions Unit 7 – Chemical Reactions Ch 11. 2 pages 330 -336

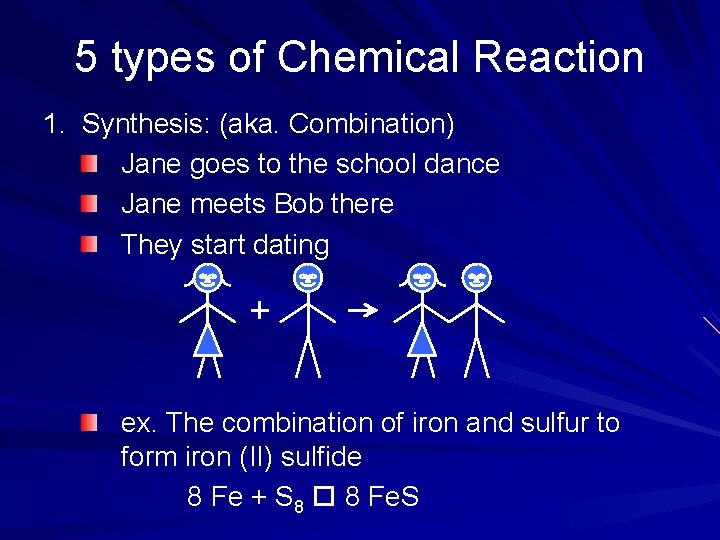
5 types of Chemical Reaction 1. Synthesis: (aka. Combination) Jane goes to the school dance Jane meets Bob there They start dating ex. The combination of iron and sulfur to form iron (II) sulfide 8 Fe + S 8 8 Fe. S

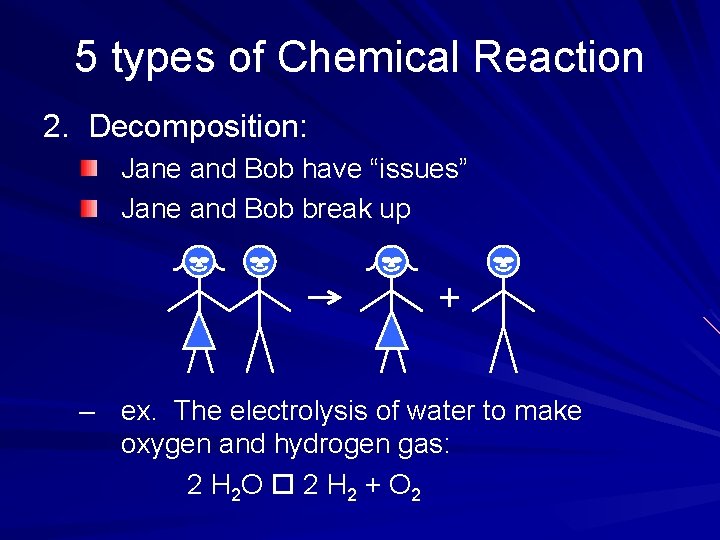
5 types of Chemical Reaction 2. Decomposition: Jane and Bob have “issues” Jane and Bob break up – ex. The electrolysis of water to make oxygen and hydrogen gas: 2 H 2 O 2 H 2 + O 2

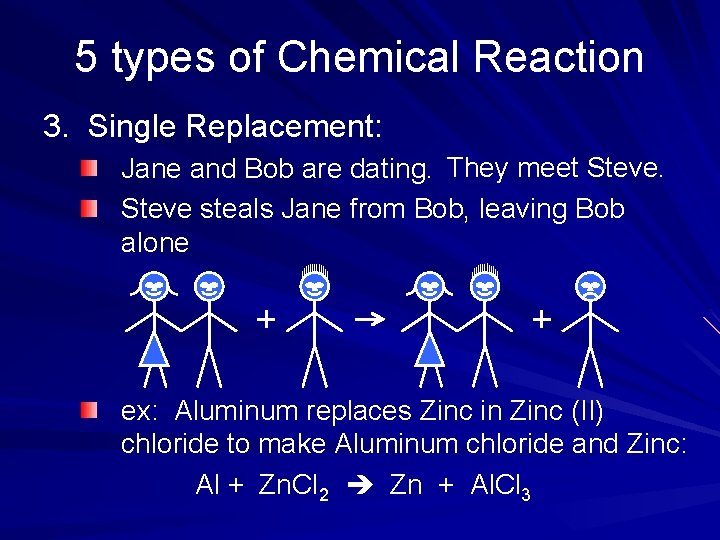
5 types of Chemical Reaction 3. Single Replacement: Jane and Bob are dating. They meet Steve steals Jane from Bob, leaving Bob alone ex: Aluminum replaces Zinc in Zinc (II) chloride to make Aluminum chloride and Zinc: Al + Zn. Cl 2 Zn + Al. Cl 3

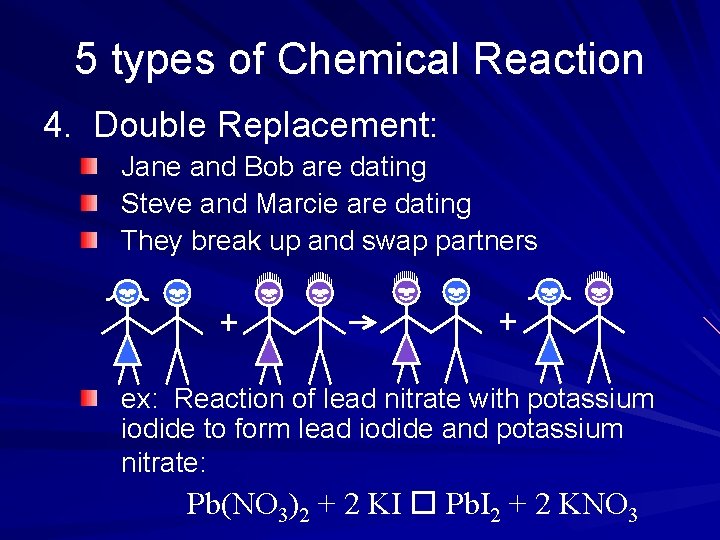
5 types of Chemical Reaction 4. Double Replacement: Jane and Bob are dating Steve and Marcie are dating They break up and swap partners ex: Reaction of lead nitrate with potassium iodide to form lead iodide and potassium nitrate: Pb(NO 3)2 + 2 KI Pb. I 2 + 2 KNO 3

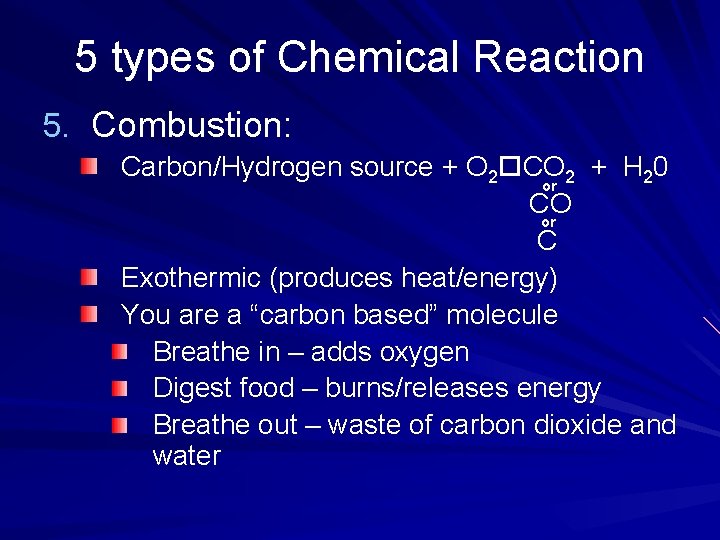
5 types of Chemical Reaction 5. Combustion: Carbon/Hydrogen source + O 2 CO 2 + H 20 or CO or C Exothermic (produces heat/energy) You are a “carbon based” molecule Breathe in – adds oxygen Digest food – burns/releases energy Breathe out – waste of carbon dioxide and water

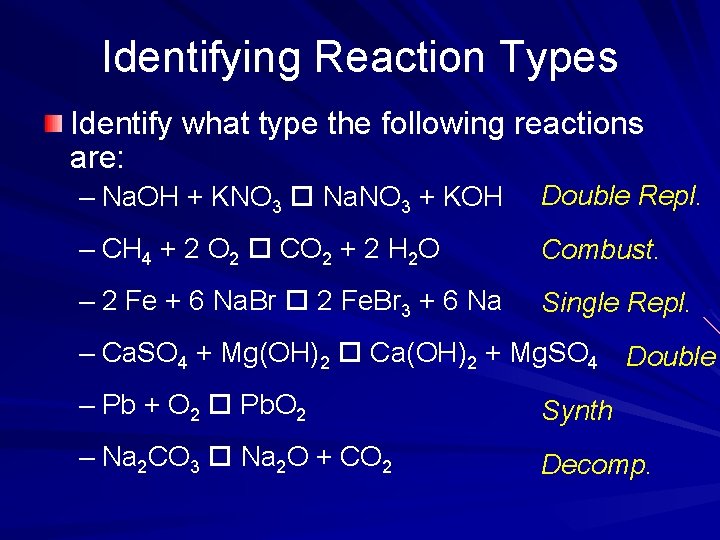
Identifying Reaction Types Identify what type the following reactions are: – Na. OH + KNO 3 Na. NO 3 + KOH Double Repl. – CH 4 + 2 O 2 CO 2 + 2 H 2 O Combust. – 2 Fe + 6 Na. Br 2 Fe. Br 3 + 6 Na Single Repl. – Ca. SO 4 + Mg(OH)2 Ca(OH)2 + Mg. SO 4 Double – Pb + O 2 Pb. O 2 Synth – Na 2 CO 3 Na 2 O + CO 2 Decomp.

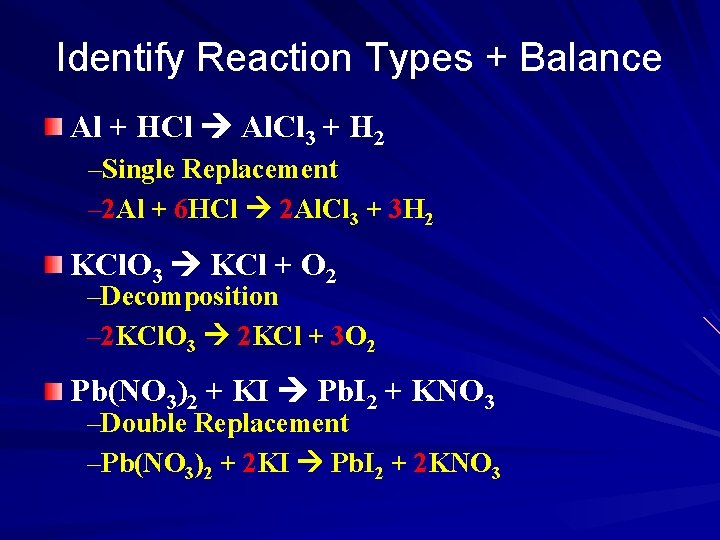
Identify Reaction Types + Balance Al + HCl Al. Cl 3 + H 2 –Single Replacement – 2 Al + 6 HCl 2 Al. Cl 3 + 3 H 2 KCl. O 3 KCl + O 2 –Decomposition – 2 KCl. O 3 2 KCl + 3 O 2 Pb(NO 3)2 + KI Pb. I 2 + KNO 3 –Double Replacement –Pb(NO 3)2 + 2 KI Pb. I 2 + 2 KNO 3

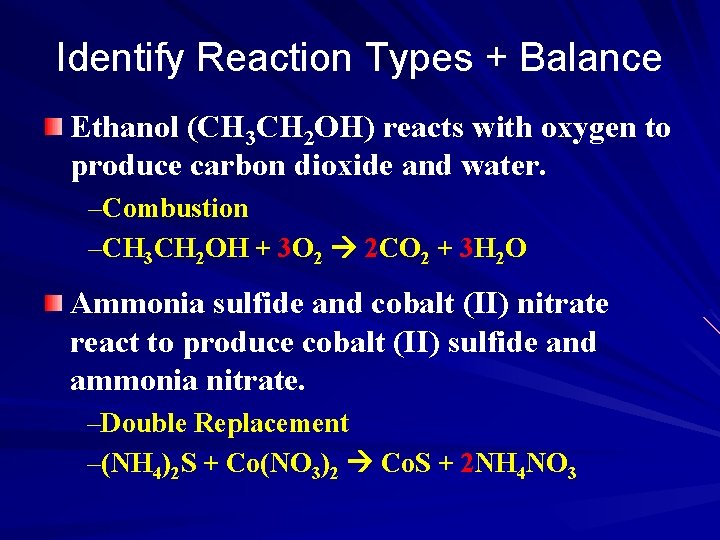
Identify Reaction Types + Balance Ethanol (CH 3 CH 2 OH) reacts with oxygen to produce carbon dioxide and water. –Combustion –CH 3 CH 2 OH + 3 O 2 2 CO 2 + 3 H 2 O Ammonia sulfide and cobalt (II) nitrate react to produce cobalt (II) sulfide and ammonia nitrate. –Double Replacement –(NH 4)2 S + Co(NO 3)2 Co. S + 2 NH 4 NO 3