Chemical Reactions Chemical Equations Chemical Reactions Chemical Change




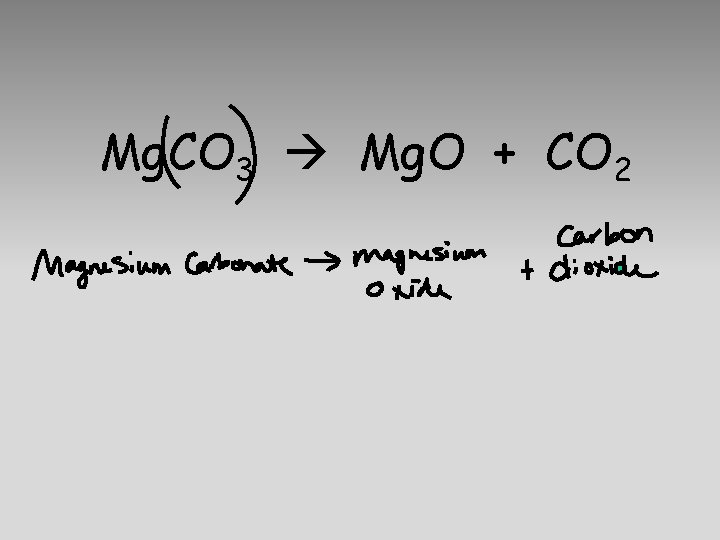
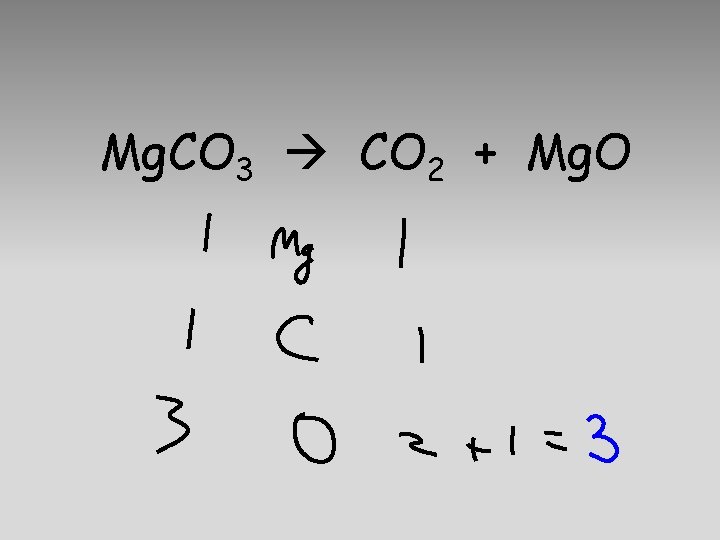















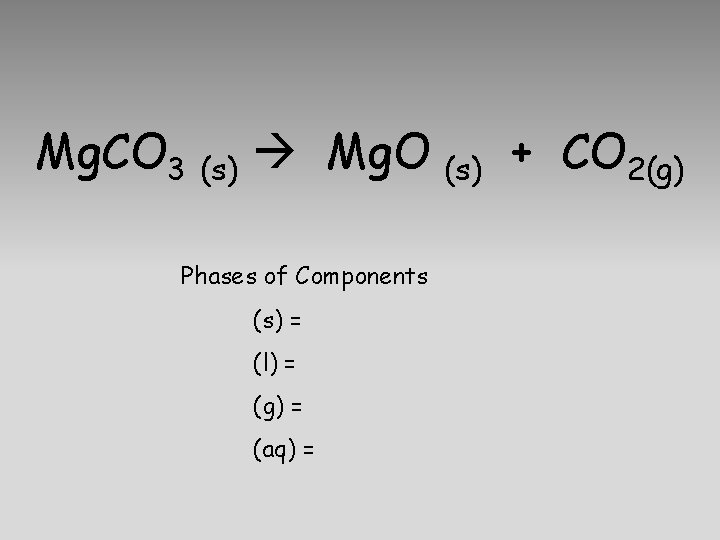
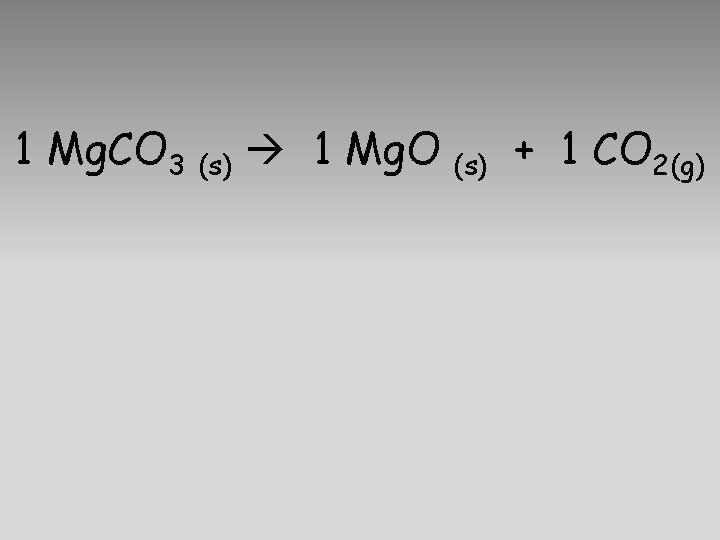



- Slides: 54

Chemical Reactions & Chemical Equations

Chemical Reactions Chemical Change A process involving a substance or substances changing into a new substance or substances

Chemical Reactions Evidence of a chemical reaction

Chemical Reactions Evidence of a chemical reaction 1) A permanent color change 2) A gas is produced 3) Energy is exchanged 4) A precipitate is produced

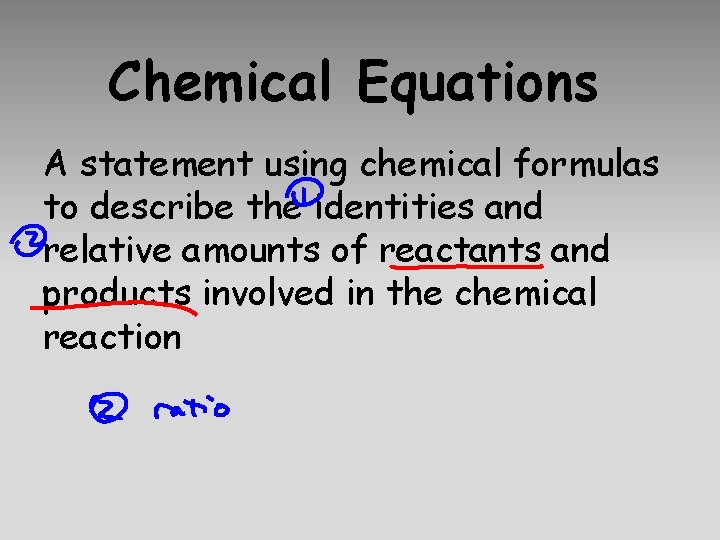
Chemical Equations A statement using chemical formulas to describe the identities and relative amounts of reactants and products involved in the chemical reaction

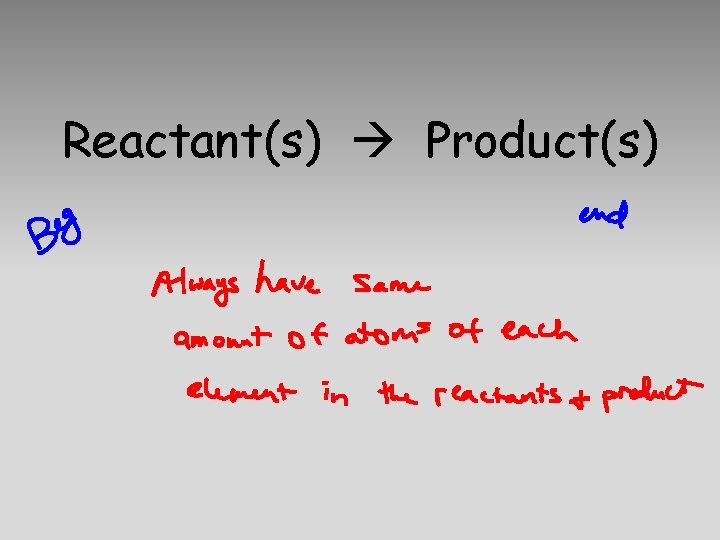
Reactant(s) Product(s)
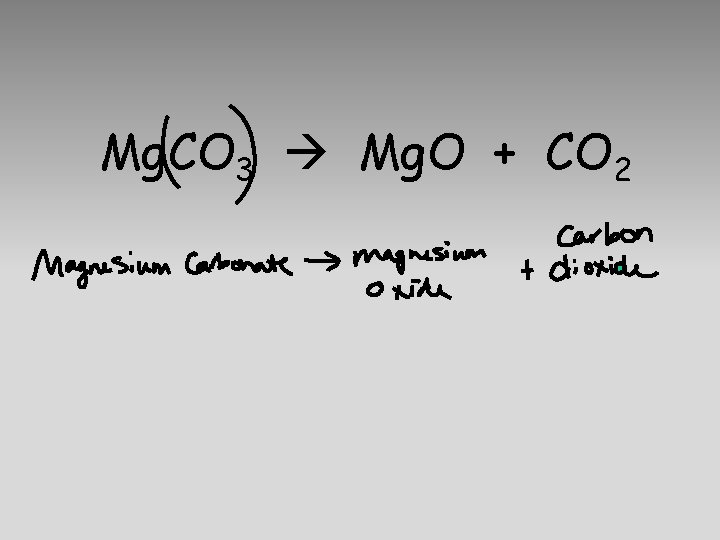
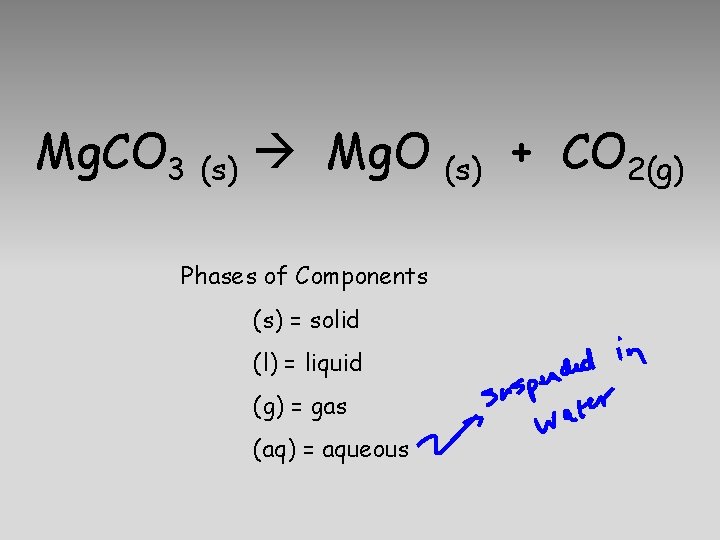
Mg. CO 3 Mg. O + CO 2
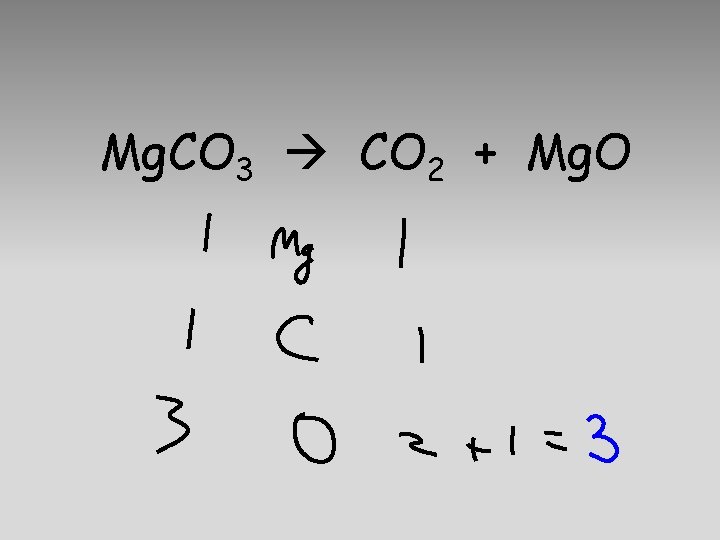
Mg. CO 3 CO 2 + Mg. O

Mg. CO 3 (s) Mg. O Phases of Components (s) = solid (l) = liquid (g) = gas (aq) = aqueous (s) + CO 2(g)

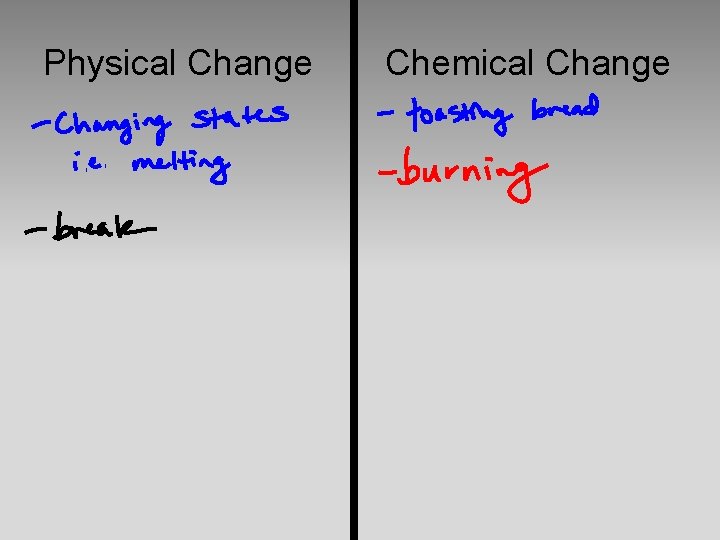
Physical Change Vs. Chemical Change

Physical Change Chemical Change

Physical Change Chemical Change Phase Changes Rearrange Atoms Dissolving New Substances form Mixing Breaking Separating Filtering

Did a chemical change occur? 1. Permanent Color Change Occurs 2. Precipitate Formed 3. Gas is Released 4. Energy is Exchanged – Temperature

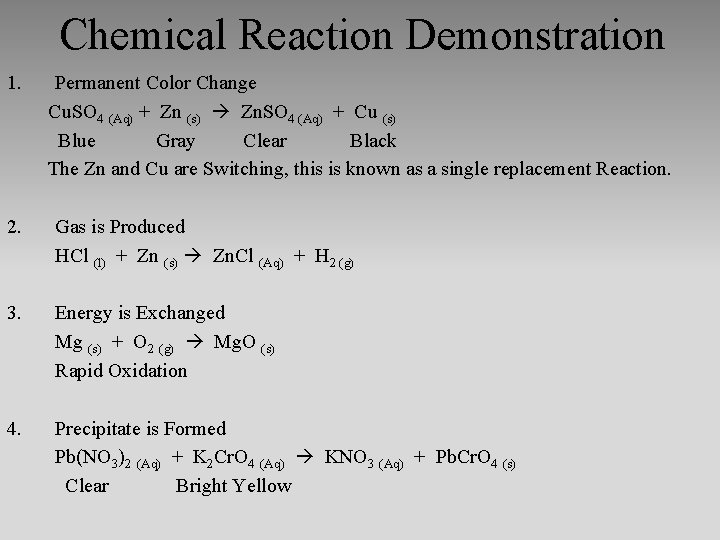
Chemical Reaction Demonstration 1. Permanent Color Change Cu. SO 4 (Aq) + Zn (s) Zn. SO 4 (Aq) + Cu (s) Blue Gray Clear Black The Zn and Cu are Switching, this is known as a single replacement Reaction. 2. Gas is Produced HCl (l) + Zn (s) Zn. Cl (Aq) + H 2 (g) 3. Energy is Exchanged Mg (s) + O 2 (g) Mg. O (s) Rapid Oxidation 4. Precipitate is Formed Pb(NO 3)2 (Aq) + K 2 Cr. O 4 (Aq) KNO 3 (Aq) + Pb. Cr. O 4 (s) Clear Bright Yellow

Practice Exercise #1 1. Define Chemical Reaction 2. How do we know a chemical reaction has taken place? 3. What is a Chemical Equation and Why do we use them? 4. What are the initial constituents of a chemical reaction? What are the end products called? 5. What do the following symbols stand for? s l g aq

Types of Chemical Equations

Synthesis • Putting things together • Also known as Direct Combination. 2 Na + Cl 2 = 2 Na. Cl 4 Al + 3 O 2 = 2 Al 2 O 3

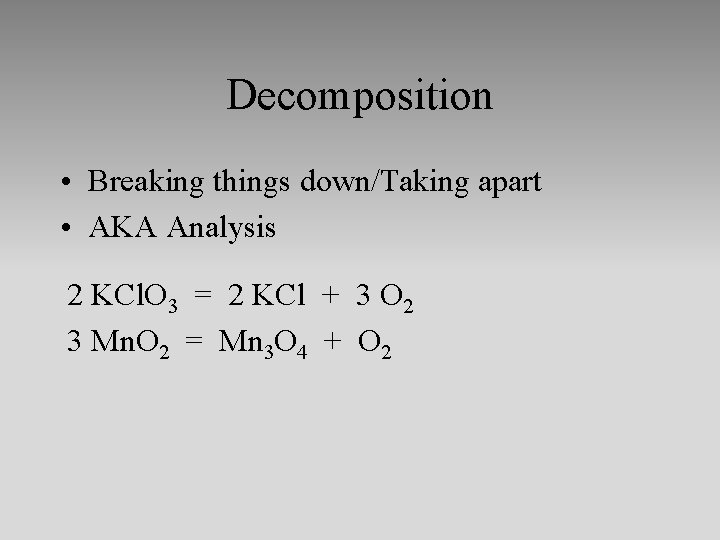
Decomposition • Breaking things down/Taking apart • AKA Analysis 2 KCl. O 3 = 2 KCl + 3 O 2 3 Mn. O 2 = Mn 3 O 4 + O 2

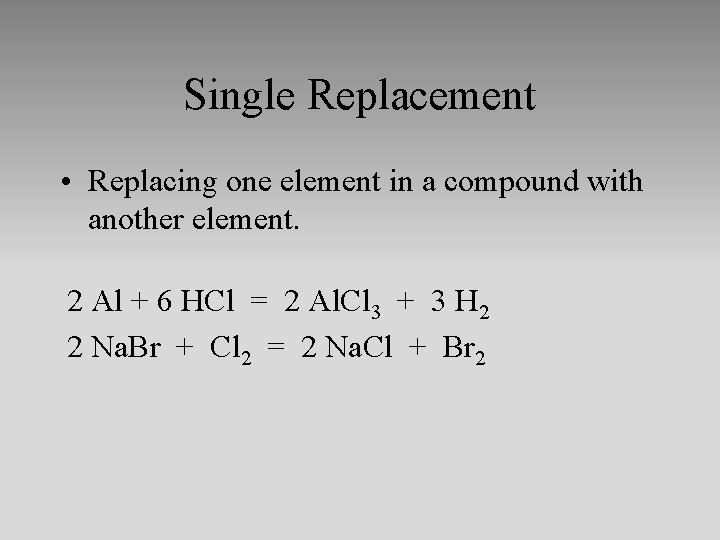
Single Replacement • Replacing one element in a compound with another element. 2 Al + 6 HCl = 2 Al. Cl 3 + 3 H 2 2 Na. Br + Cl 2 = 2 Na. Cl + Br 2

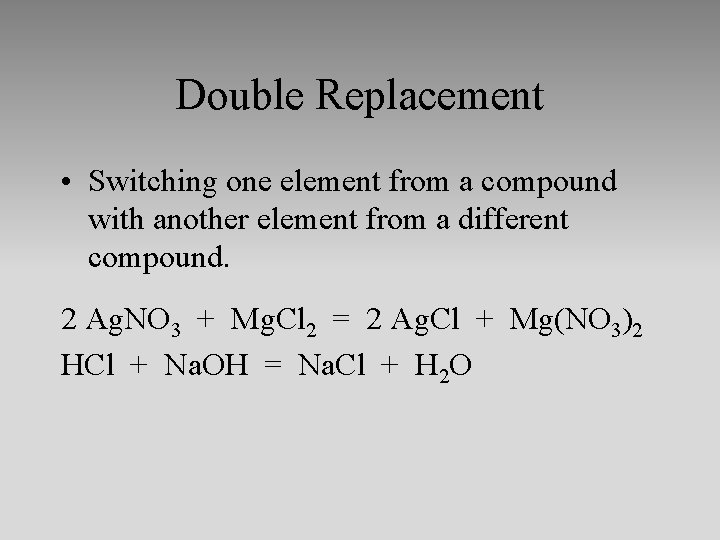
Double Replacement • Switching one element from a compound with another element from a different compound. 2 Ag. NO 3 + Mg. Cl 2 = 2 Ag. Cl + Mg(NO 3)2 HCl + Na. OH = Na. Cl + H 2 O

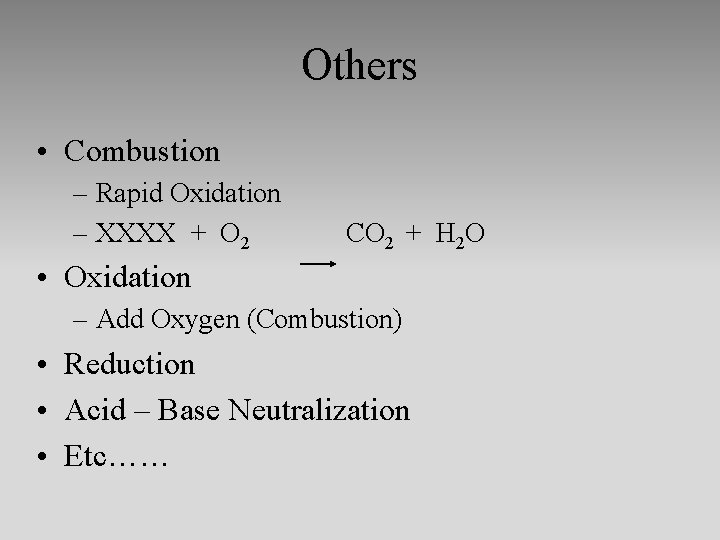
Others • Combustion – Rapid Oxidation – XXXX + O 2 CO 2 + H 2 O • Oxidation – Add Oxygen (Combustion) • Reduction • Acid – Base Neutralization • Etc……


HW • Due Monday • Read & outline page 249 -255

Exothermic Reactions • Release or give off heat. • Heat is a byproduct of the reaction. • Temperature increases as the bonds between products are formed. • Examples – Combustion, Respiration, Oxidation

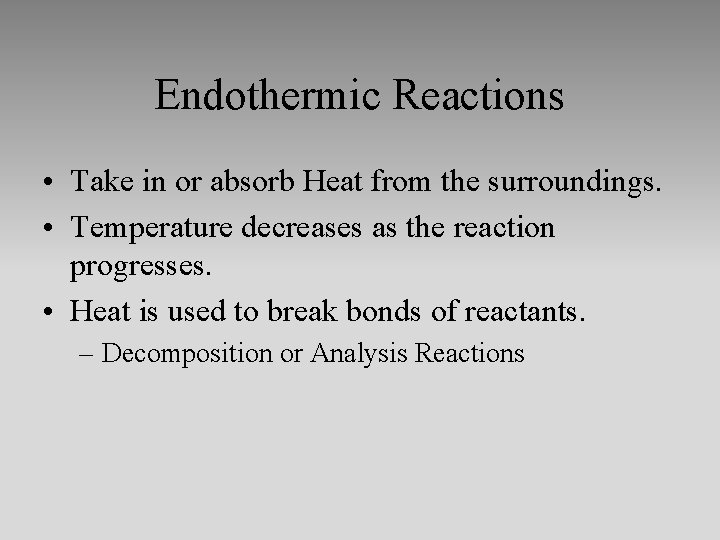
Endothermic Reactions • Take in or absorb Heat from the surroundings. • Temperature decreases as the reaction progresses. • Heat is used to break bonds of reactants. – Decomposition or Analysis Reactions

Oxidation • Removing Electrons

Reduction • Adding Electrons

Rate of Reaction • Reactions occur at different speeds. • The time it takes for reactants to become products.

Catalysts • They speed up reactions without being affected themselves. – They stay the same – Enzymes in living organisms. • Lowers activation energy – The amount of energy needed to start a reaction.

Speeding Up Reactions • Temperature, Energy, Pressure, Surface Area, Concentration, Collisions, Catalyst.

Measuring Rates of Reactions • Measure Reactants • Measure Products

Balancing Chemical Equations

Reactant(s) Product(s)
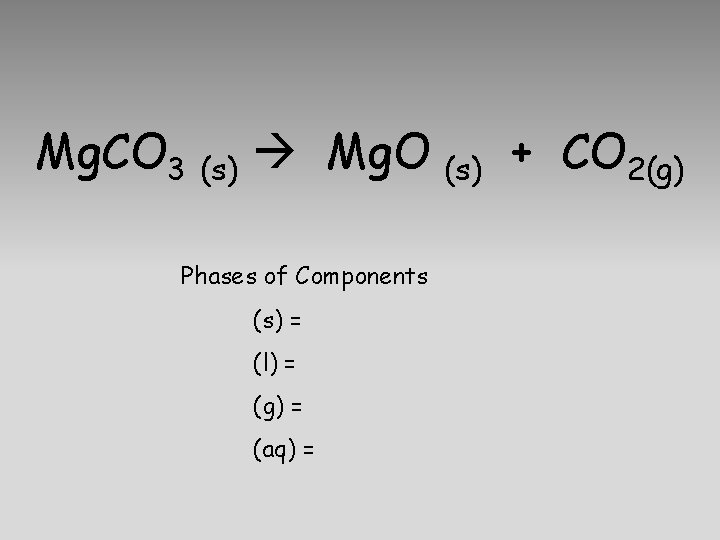
Mg. CO 3 (s) Mg. O Phases of Components (s) = (l) = (g) = (aq) = (s) + CO 2(g)
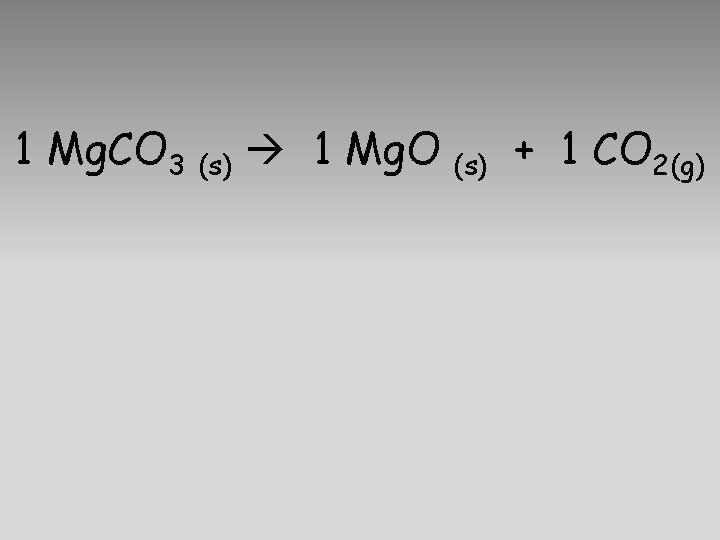
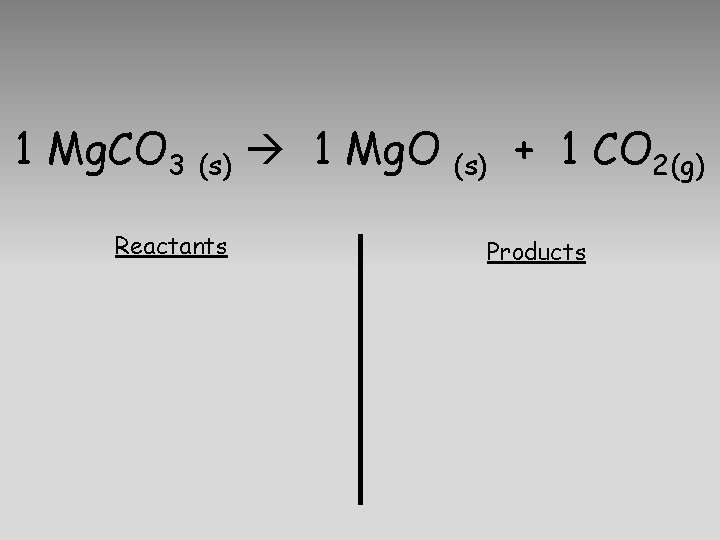
1 Mg. CO 3 (s) 1 Mg. O (s) + 1 CO 2(g)

1 Mg. CO 3 (s) 1 Mg. O Reactants (s) + 1 CO 2(g) Products

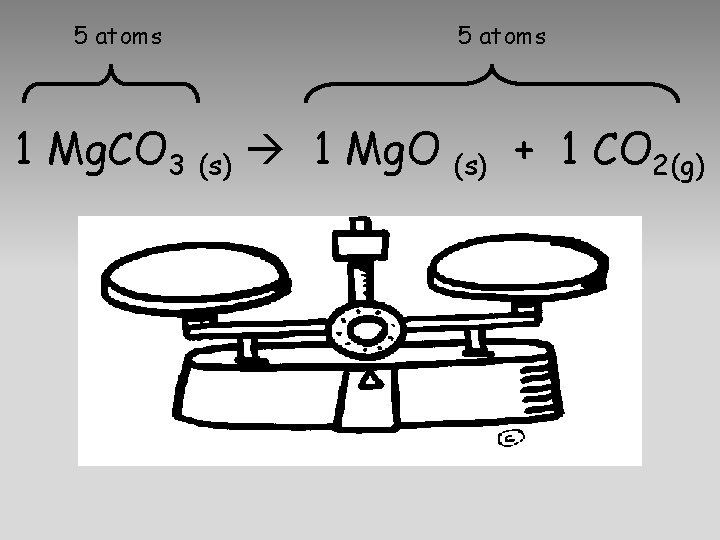
5 atoms 1 Mg. CO 3 (s) 1 Mg. O 5 atoms (s) + 1 CO 2(g)

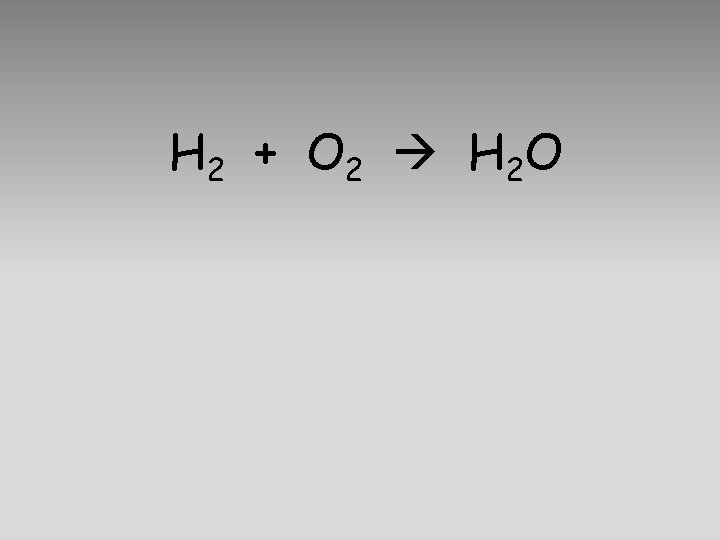
H 2 + O 2 H 2 O

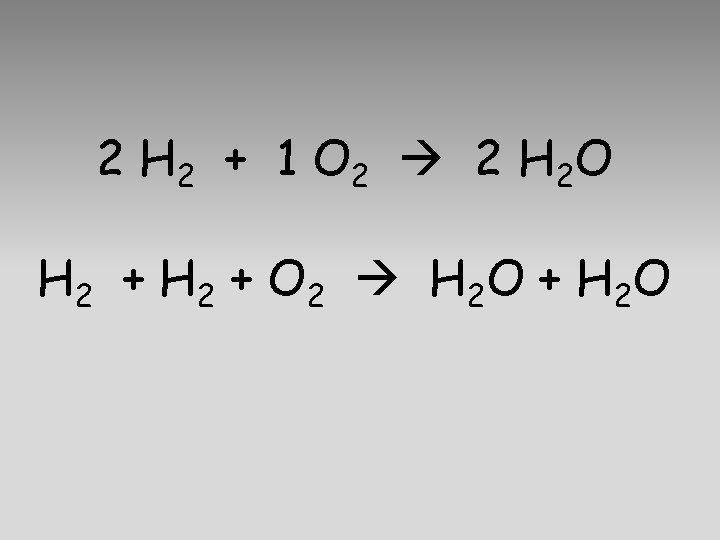
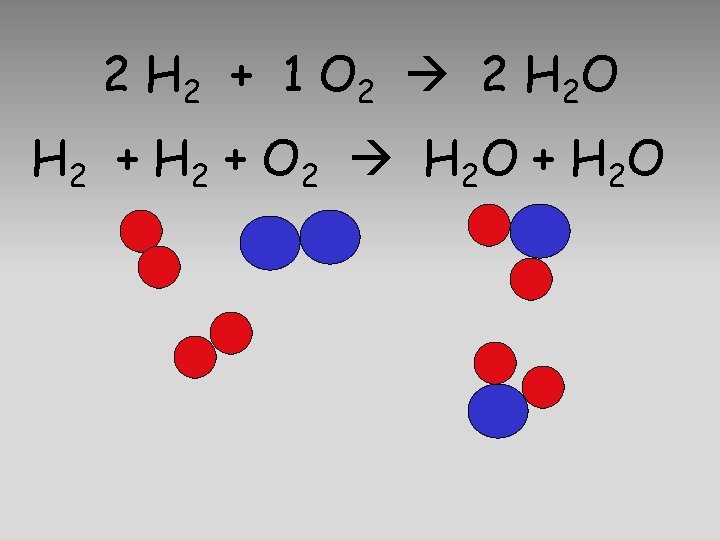
2 H 2 + 1 O 2 2 H 2 O H 2 + O 2 H 2 O + H 2 O

2 H 2 + 1 O 2 2 H 2 O H 2 + O 2 H 2 O + H 2 O

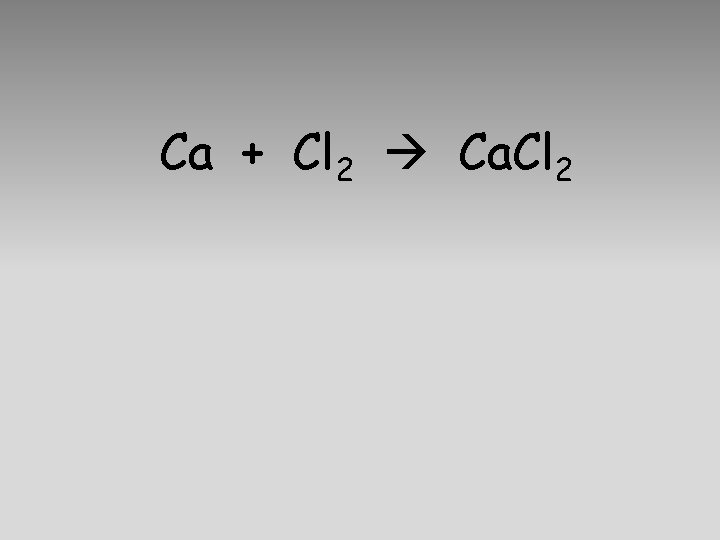
Ca + Cl 2 Ca. Cl 2

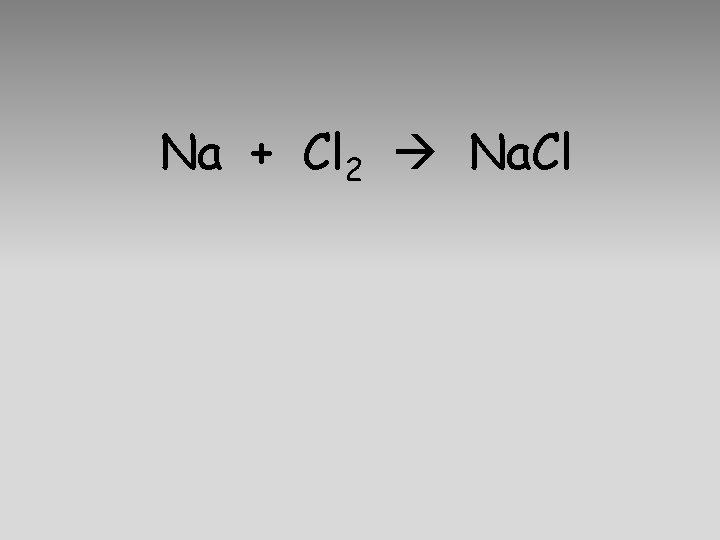
Na + Cl 2 Na. Cl

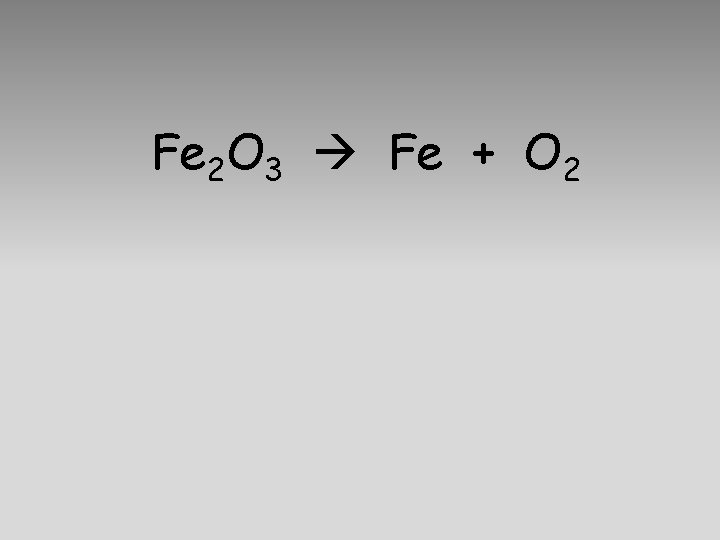
Fe 2 O 3 Fe + O 2

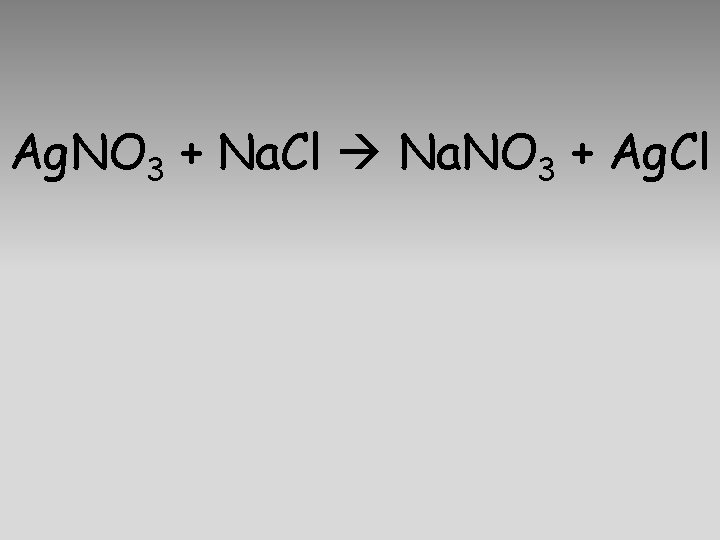
Ag. NO 3 + Na. Cl Na. NO 3 + Ag. Cl

Fe. S 2 + O 2 Fe 2 O 3 + SO 2

4 Fe. S 2 + 11 O 2 2 Fe 2 O 3 + 8 SO 2

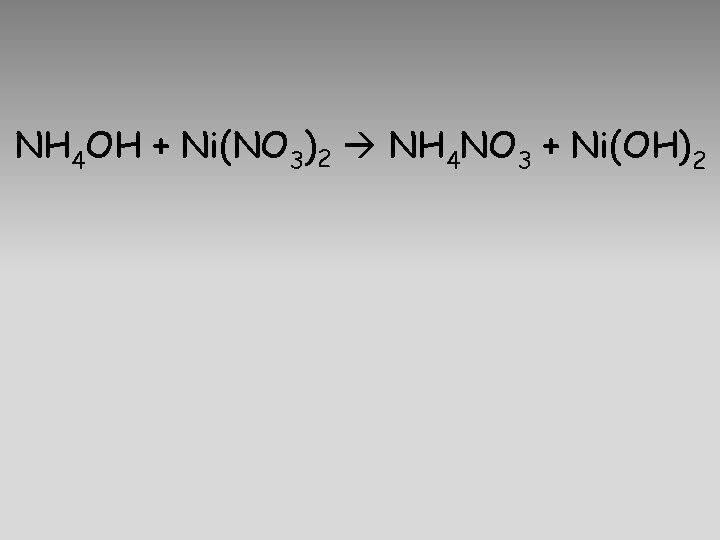
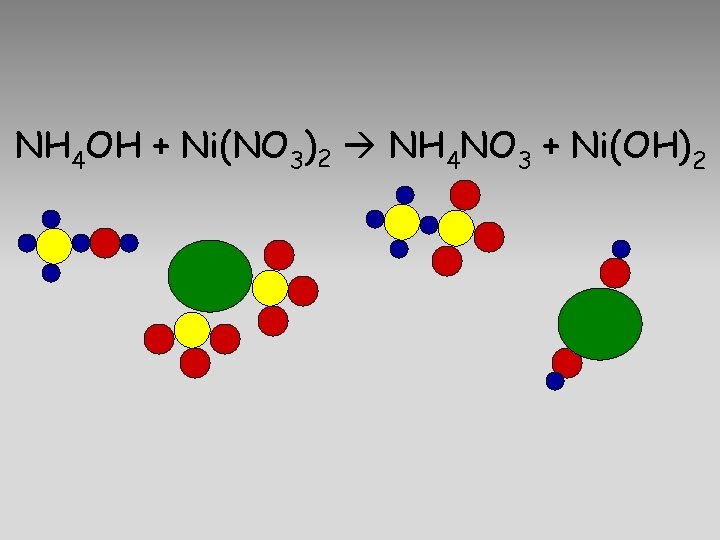
NH 4 OH + Ni(NO 3)2 NH 4 NO 3 + Ni(OH)2

NH 4 OH + Ni(NO 3)2 NH 4 NO 3 + Ni(OH)2

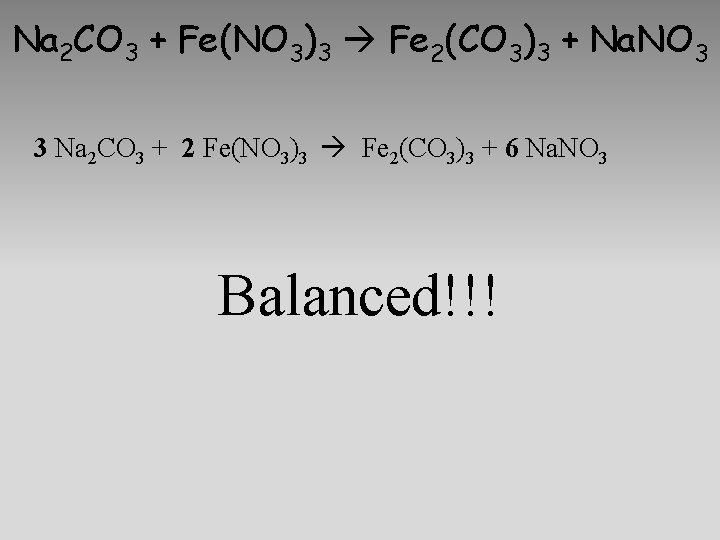
Na 2 CO 3 + Fe(NO 3)3 Fe 2(CO 3)3 + Na. NO 3

Na 2 CO 3 + Fe(NO 3)3 Fe 2(CO 3)3 + Na. NO 3 3 Na 2 CO 3 + 2 Fe(NO 3)3 Fe 2(CO 3)3 + 6 Na. NO 3 Balanced!!!

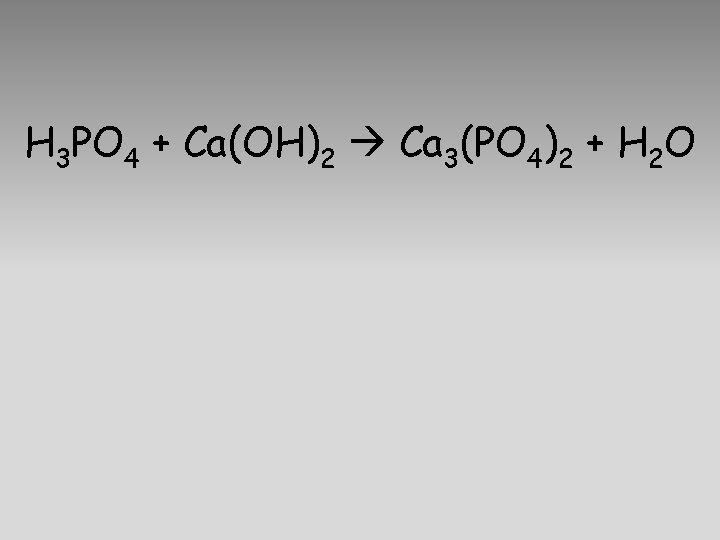
H 3 PO 4 + Ca(OH)2 Ca 3(PO 4)2 + H 2 O

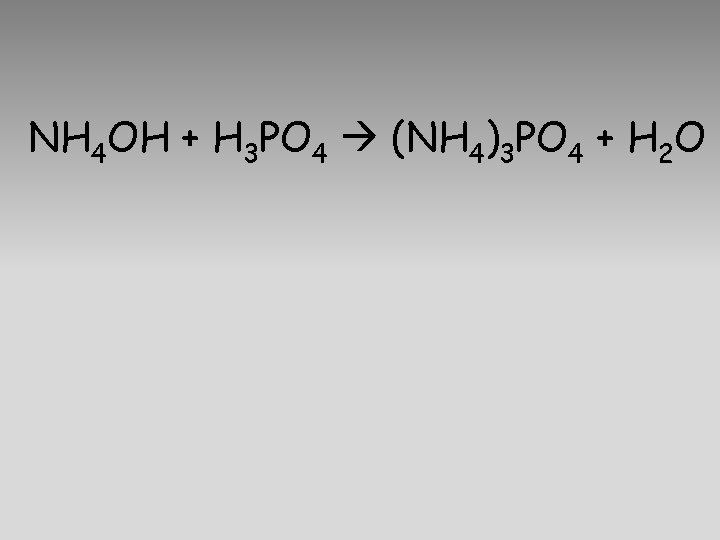
NH 4 OH + H 3 PO 4 (NH 4)3 PO 4 + H 2 O

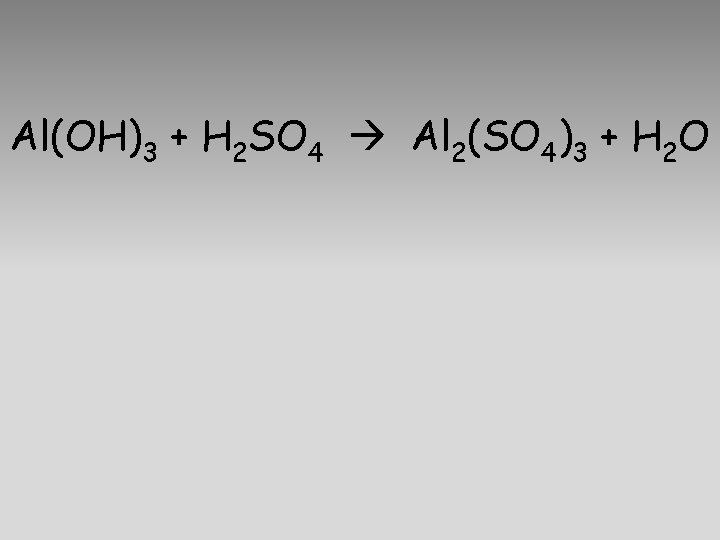
Al(OH)3 + H 2 SO 4 Al 2(SO 4)3 + H 2 O

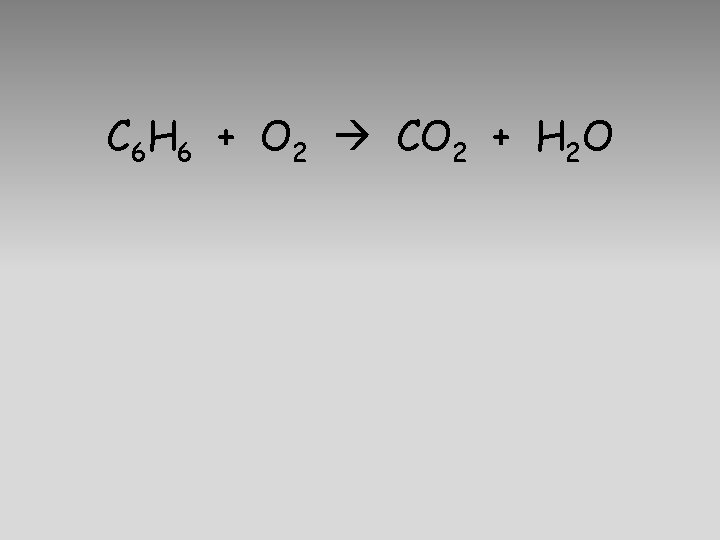
C 6 H 6 + O 2 CO 2 + H 2 O