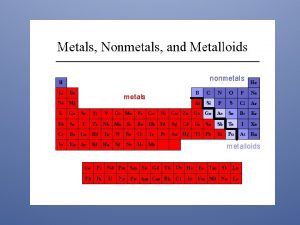
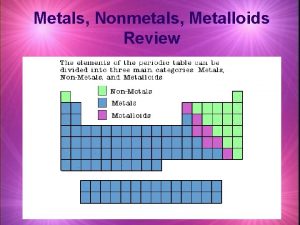
Metals Nonmetals and Metalloids Coloring in the Periodic




















- Slides: 36

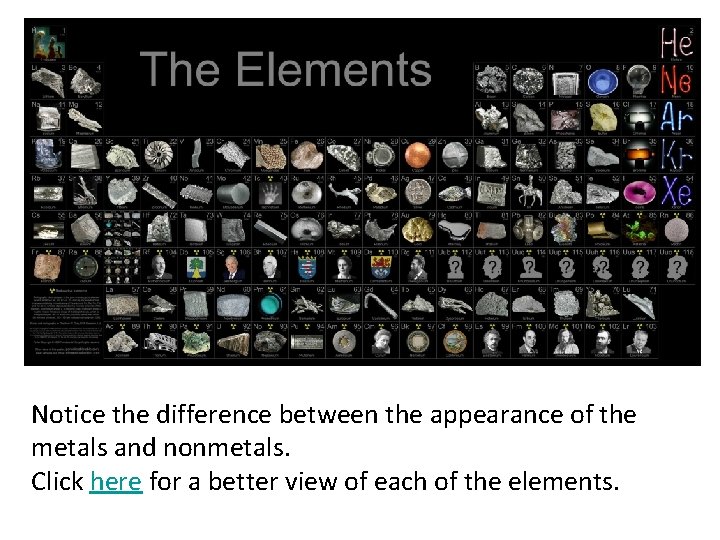
Metals, Nonmetals and Metalloids

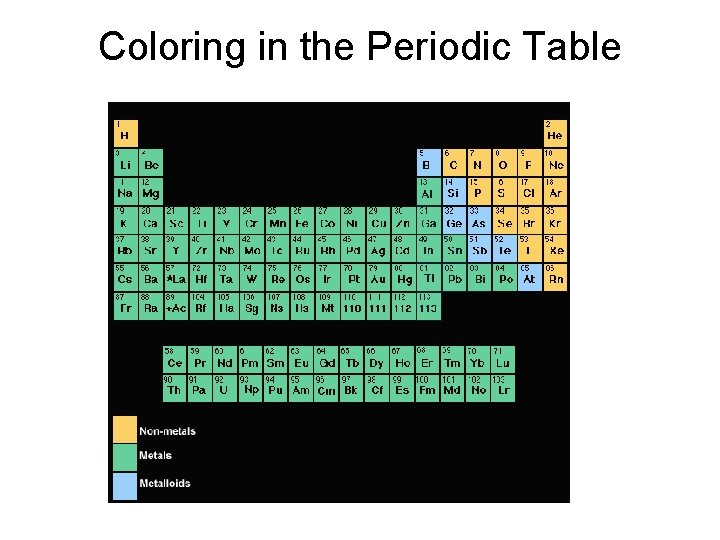
Coloring in the Periodic Table

Notice the difference between the appearance of the metals and nonmetals. Click here for a better view of each of the elements.

• • • Physical Properties of Metals: Luster (shininess) Good conductors of heat and electricity High density (heavy for their size) High melting point Ductile (most metals can be drawn out into thin wires) Malleable (most metals can be hammered into thin sheets) Chemical Properties of Metals: Easily lose electrons Bismuth Corrode easily. Corrosion is a gradual wearing away. (Example: silver tarnishing and iron rusting


Physical Properties of METALS Metals are malleable. metals ability to be shaped or formed as by hammering or pressure; can be beaten into thin sheets Aluminum is malleab

Physical Properties of METALS Metals have shiny luster. (or metallic luster) LUSTER – the way an object’s surface reflects light

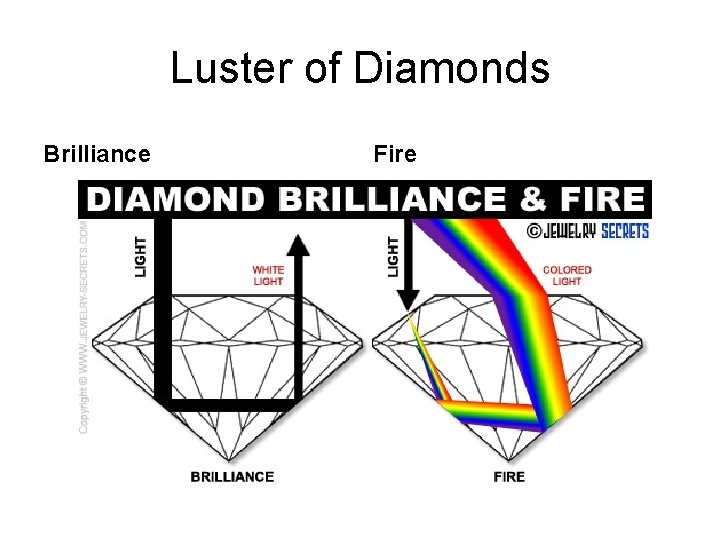
Luster of Diamonds Brilliance Fire

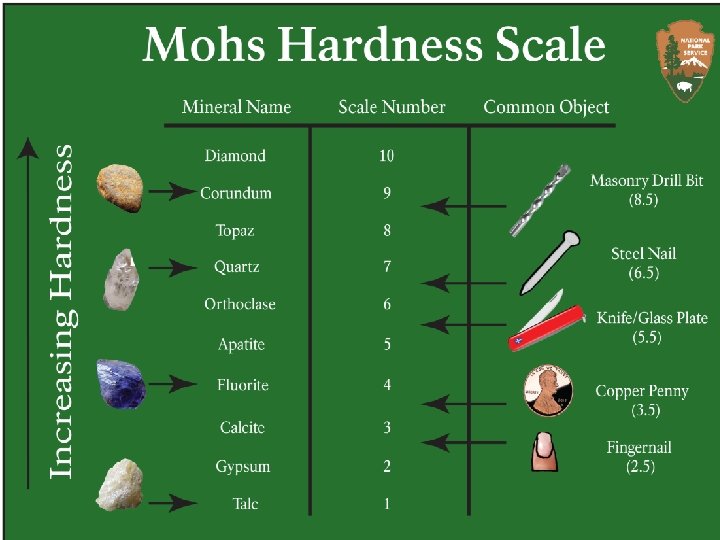
Physical properties of METALS • Metals are SOLIDS. (except mercury) • Metals are HARD. (except Lithium, Potassium, Sodium)


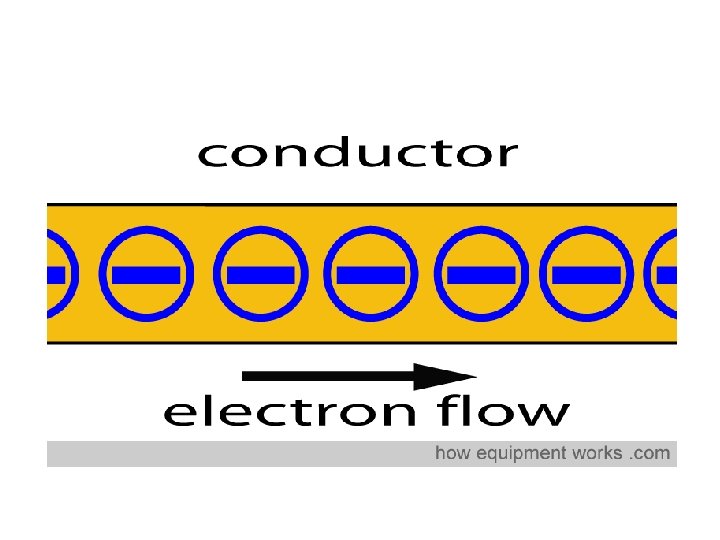
Physical Properties of METALS • Metals are good conductors of electricity. In a conductor, electric current can flow freely. Copper Wiring Copper, silver and gold are good electrical conductors!

Conductors electrons free to roam


METALS are the best conductors of heat. Electrons in metals move more freely, allowing heat energy to travel across the metal. Think about whenever you’ve left a spoon in a hot drink! Best conductors: silver and copper

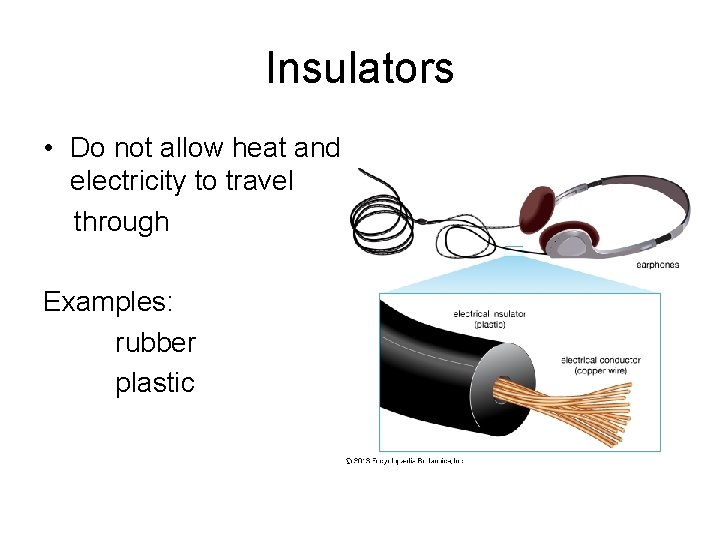
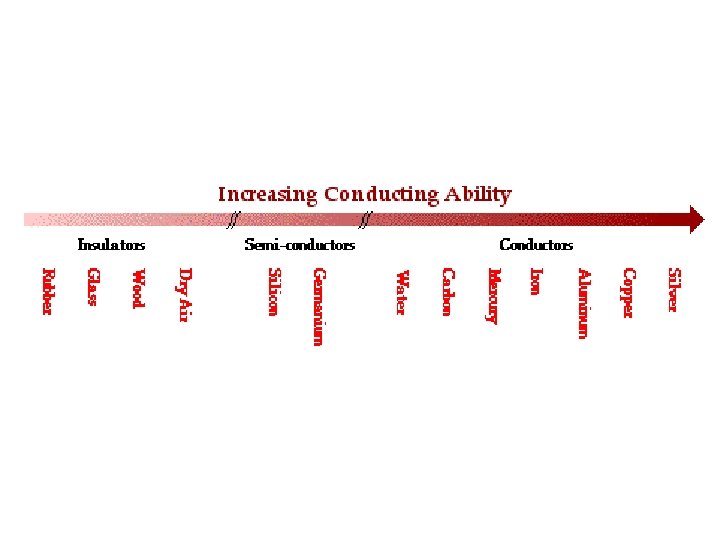
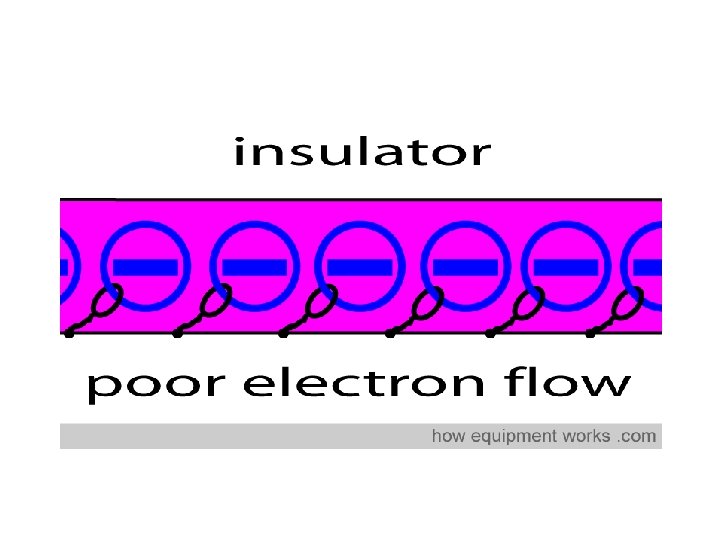
Insulators • Do not allow heat and electricity to travel through Examples: rubber plastic



Insulators – electrons don’t transfer energy

Physical Properties of METALS Metals are ductile. Ductility or ductile – can be drawn into a wire


Physical Properties of Metals: • • • Luster (shininess) Good conductors of heat and electricity High density (heavy for their size) High melting point Ductile Malleable Chemical Properties of Metals: • Easily lose electrons • Corrode easily. Corrosion is a gradual wearing away. (Example: silver tarnishing and iron rusting

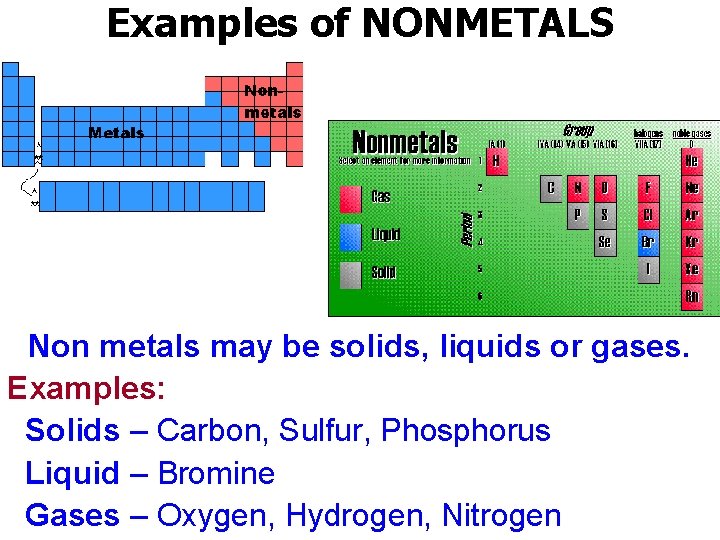
Examples of NONMETALS Non metals may be solids, liquids or gases. Examples: Solids – Carbon, Sulfur, Phosphorus Liquid – Bromine Gases – Oxygen, Hydrogen, Nitrogen

Physical Properties of NONMETALS Nonmetals have a dull luster. (They are not shiny!) Ex. Phosphorus

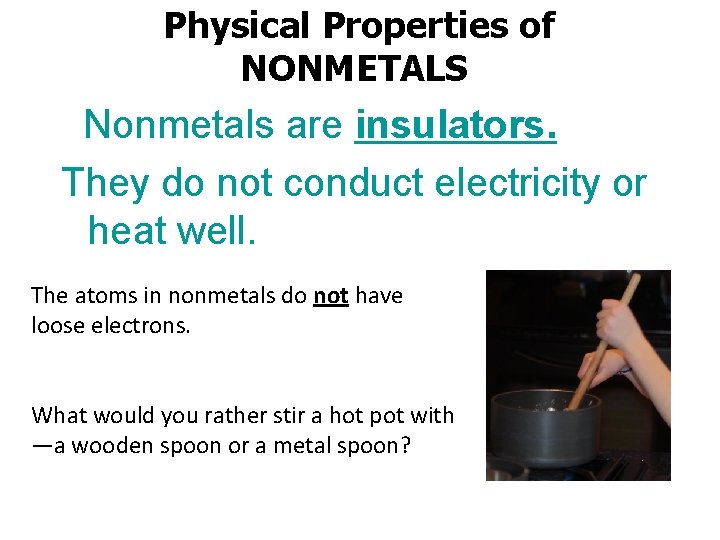
Physical Properties of NONMETALS Nonmetals are insulators. They do not conduct electricity or heat well. The atoms in nonmetals do not have loose electrons. What would you rather stir a hot pot with —a wooden spoon or a metal spoon?

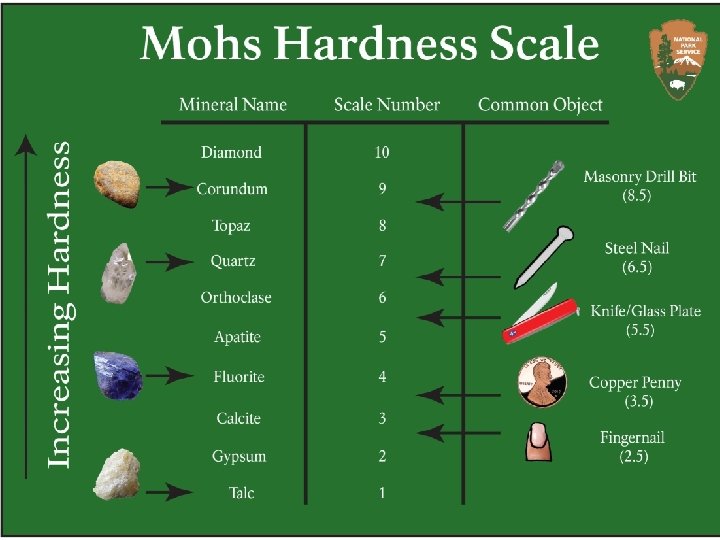
Physical Properties of NONMETALS Nonmetals are soft (except for diamonds) and brittle. Example: Sulfur


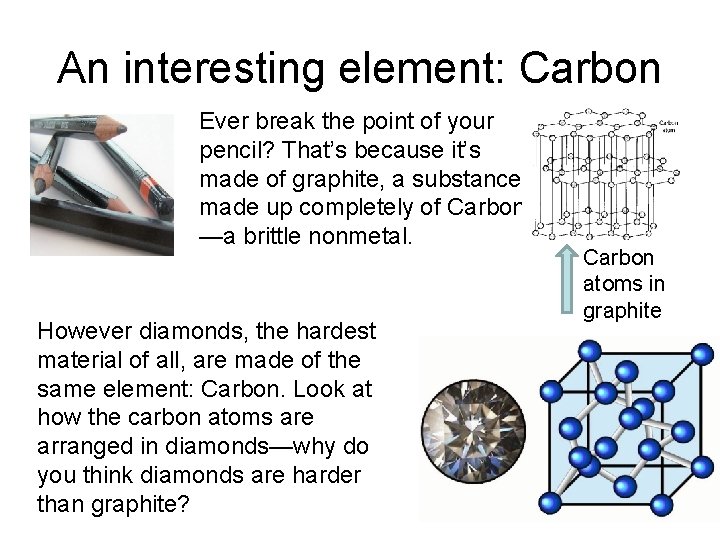
An interesting element: Carbon Ever break the point of your pencil? That’s because it’s made of graphite, a substance made up completely of Carbon —a brittle nonmetal. However diamonds, the hardest material of all, are made of the same element: Carbon. Look at how the carbon atoms are arranged in diamonds—why do you think diamonds are harder than graphite? Carbon atoms in graphite

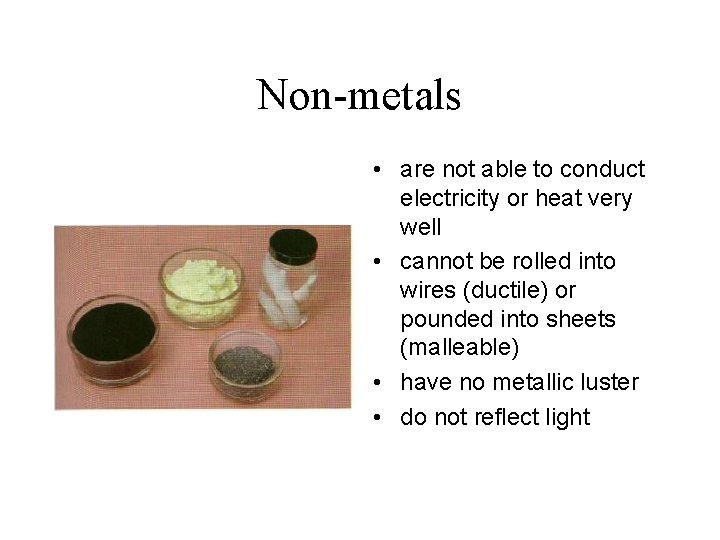
Non-metals • are not able to conduct electricity or heat very well • cannot be rolled into wires (ductile) or pounded into sheets (malleable) • have no metallic luster • do not reflect light

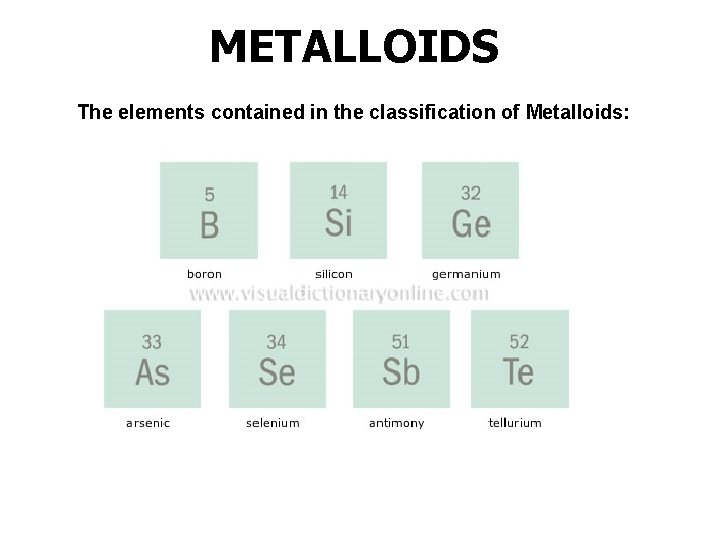
METALLOIDS The elements contained in the classification of Metalloids:

METALLOIDS • Physical properties of both metals and nonmetals. • Some are shiny, some are dull, they are somewhat malleable and ductile, and can conduct heat and electricity at a lesser level than metals. SILICON BORON ARSENIC

METALLOIDS • Some metalloids are useful semiconductors, which are used in electronics (radio, computers, telephones, etc. ) • They are useful because they conduct just the right amount of electricity or heat.

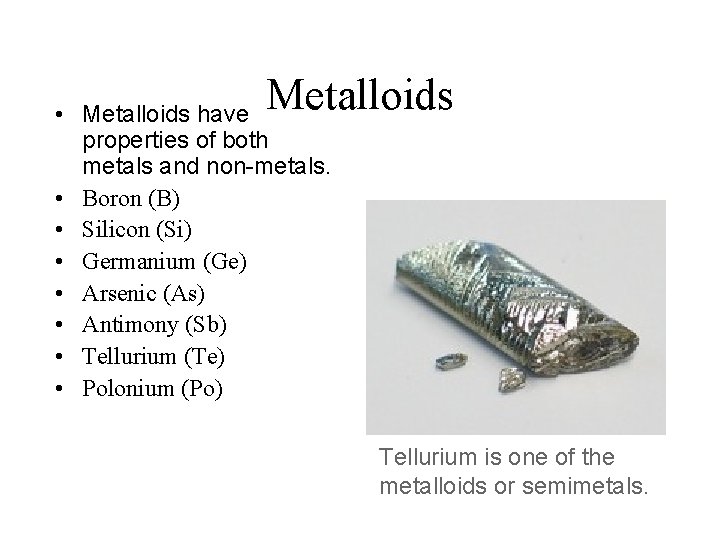
Metalloids • Metalloids have properties of both metals and non-metals. • Boron (B) • Silicon (Si) • Germanium (Ge) • Arsenic (As) • Antimony (Sb) • Tellurium (Te) • Polonium (Po) Tellurium is one of the metalloids or semimetals.

Where do we find METALS? Some metals like gold, silver, and platinum are found as pure substances in the earth’s crust because they are least reactive. Most metals are reactive and are found as oxides (react with oxygen), carbonates (react with carbon), sulfides (react with sulfur). Minerals : are elements or compounds which occur naturally inside the earth’s crust. Ore : is a mineral from which metals can be extracted profitably.

Video on Physical Properties of Metals • https: //www. youtube. com/watch? v=5 R 08 N 3 u 5 Z_Y • https: //www. youtube. com/watch? v=Oz 8 Gp DVz 5 ag

How coins are made • http: //www. youtube. com/watch? v=Dk. HFN n. OK 3 Bg

How aluminum foil is made • http: //www. youtube. com/watch? v=f 4 OTj 9 y NOak
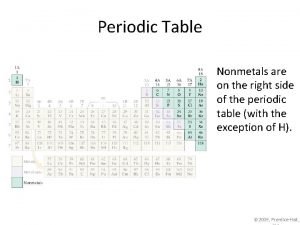
 Nonmetals on the periodic table
Nonmetals on the periodic table What separates metals from nonmetals
What separates metals from nonmetals Metals nonmetals and metalloids periodic table
Metals nonmetals and metalloids periodic table Periodic table divided in metals nonmetals and metalloids
Periodic table divided in metals nonmetals and metalloids Periodic table metals nonmetals metalloids noble gases
Periodic table metals nonmetals metalloids noble gases Labeled periodic table
Labeled periodic table Metals vs nonmetals periodic table
Metals vs nonmetals periodic table Metalloids vs metals
Metalloids vs metals Metals nonmetals and metalloids answer key
Metals nonmetals and metalloids answer key Metals vs nonmetals vs metalloids
Metals vs nonmetals vs metalloids Differentiate metals nonmetals and metalloids
Differentiate metals nonmetals and metalloids Properties of metals nonmetals and semimetals
Properties of metals nonmetals and semimetals Brittle nonmetals
Brittle nonmetals Poem about metals nonmetals and metalloids
Poem about metals nonmetals and metalloids Periodic metals and nonmetals
Periodic metals and nonmetals Least reactive non-metal
Least reactive non-metal Nonmetals on the periodic table
Nonmetals on the periodic table Non metals melting and boiling points
Non metals melting and boiling points Non metals and uses
Non metals and uses Optical properties of metals and nonmetals
Optical properties of metals and nonmetals Chapter 4 metals and nonmetals
Chapter 4 metals and nonmetals Where are metalloids located on the periodic table
Where are metalloids located on the periodic table Periodic table metalloids
Periodic table metalloids Metalloids on the periodic table
Metalloids on the periodic table Periodic table coloring page
Periodic table coloring page Metals vs nonmetals properties
Metals vs nonmetals properties Reactivity periodic table
Reactivity periodic table Are ionic compounds metals or nonmetals
Are ionic compounds metals or nonmetals Metals vs nonmetals
Metals vs nonmetals Periodic table regents
Periodic table regents Ionic compounds have
Ionic compounds have Properties of materials grade 7 powerpoint
Properties of materials grade 7 powerpoint Social science grade 7 term 2
Social science grade 7 term 2 Periodic table coloring activity key
Periodic table coloring activity key Where are the non-metals located on the periodic table
Where are the non-metals located on the periodic table Structure of atom periodic table
Structure of atom periodic table Ferrous vs non ferrous
Ferrous vs non ferrous