Metals Nonmetals and Metalloids 1 Metals Nonmetals and




![Periodic Table: 4) f-block: The outermost electron is placed in “f” orbitals. Ex. Ce-[Xe] Periodic Table: 4) f-block: The outermost electron is placed in “f” orbitals. Ex. Ce-[Xe]](https://slidetodoc.com/presentation_image_h2/52ff620e29f05368c7ce5a7bf2656f08/image-23.jpg)
- Slides: 25

Metals, Nonmetals, and Metalloids: 1

Metals, Nonmetals, and Metalloids 2

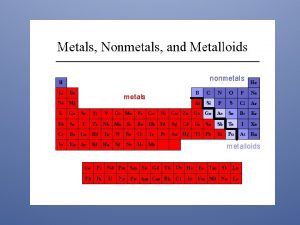
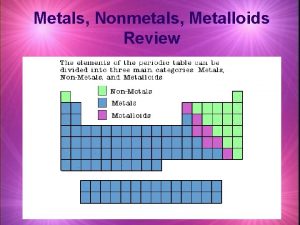
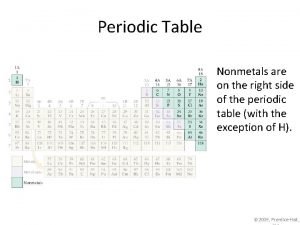
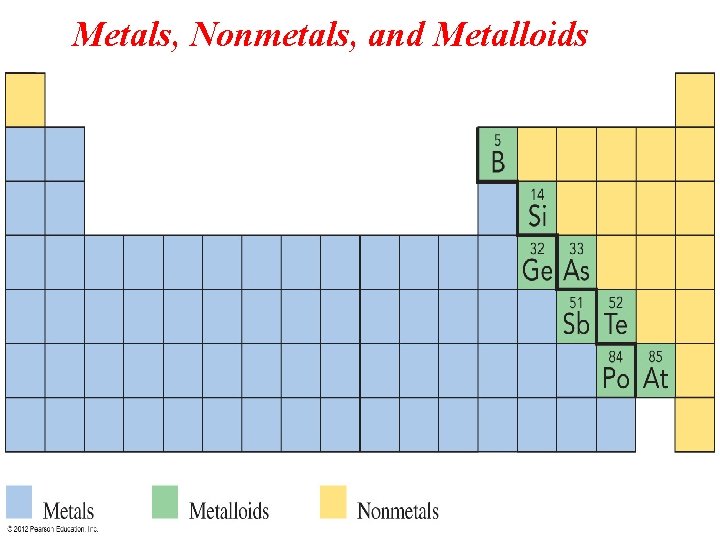
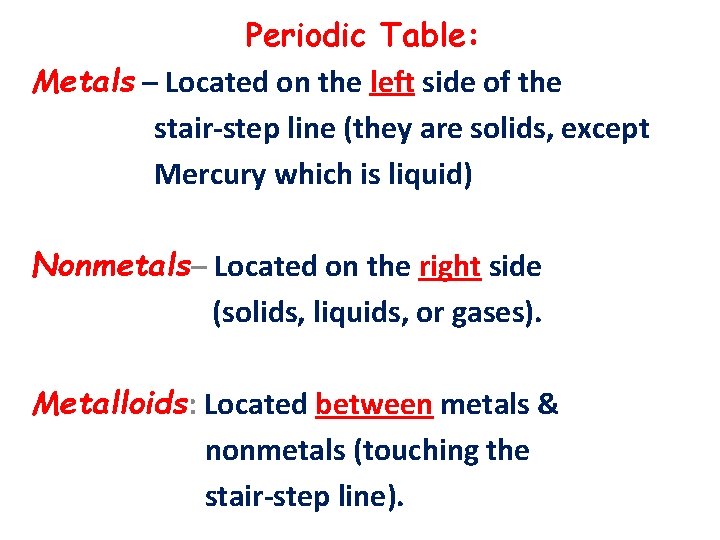
Periodic Table: Metals – Located on the left side of the stair-step line (they are solids, except Mercury which is liquid) Nonmetals– Located on the right side (solids, liquids, or gases). Metalloids: Located between metals & nonmetals (touching the stair-step line).

Metals (on the left): 1) Solids at room temperature 2) Shiny, can be converted into wires (ductile) & into sheets (malleable). 3) High melting & boiling points. 4) Good conductors of electricity & heat.

Non metals (on the right): 1) Solids, liquids, or gases at room temp. 2) Low melting & boiling points. 3) They are brittle & poor conductors of electricity & heat. Metalloids: They have properties of both metals & non metals.

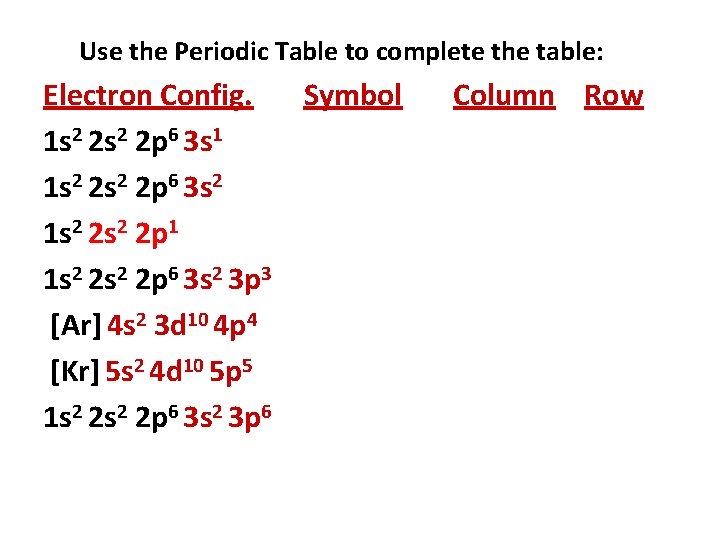
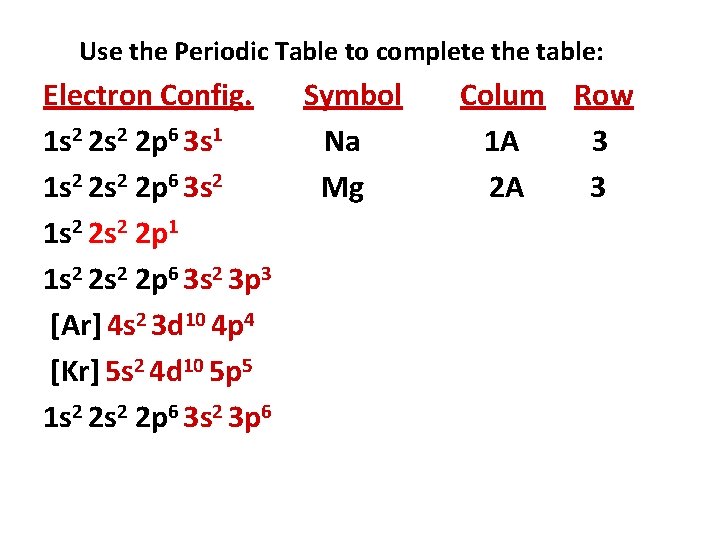
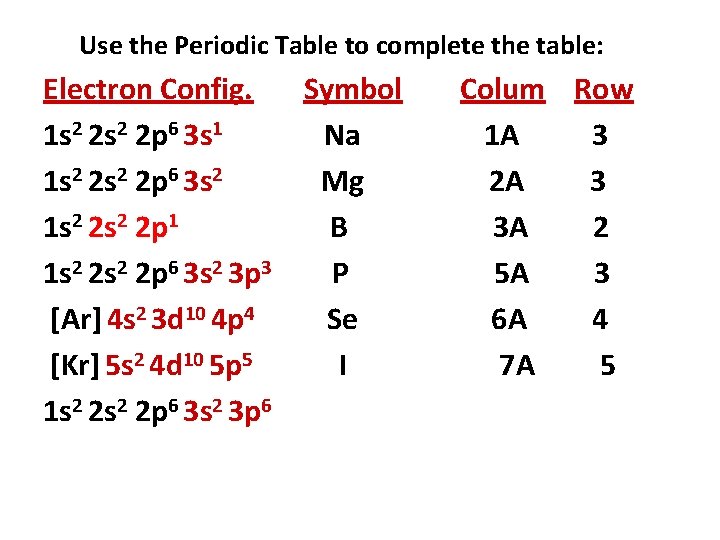
Use the Periodic Table to complete the table: Electron Config. Symbol 1 s 2 2 p 6 3 s 1 1 s 2 2 p 6 3 s 2 1 s 2 2 p 1 1 s 2 2 p 6 3 s 2 3 p 3 [Ar] 4 s 2 3 d 10 4 p 4 [Kr] 5 s 2 4 d 10 5 p 5 1 s 2 2 p 6 3 s 2 3 p 6 Column Row

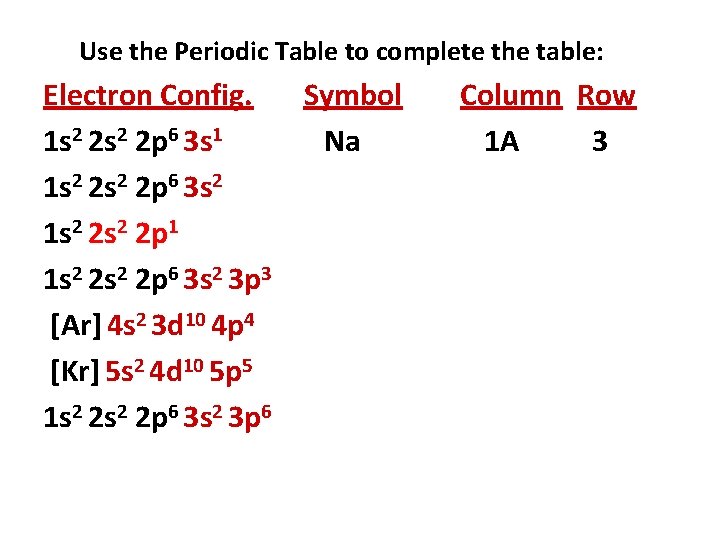
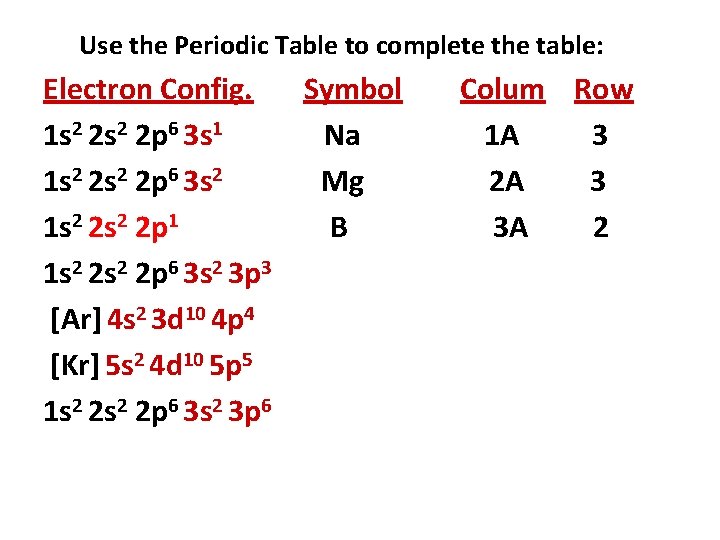
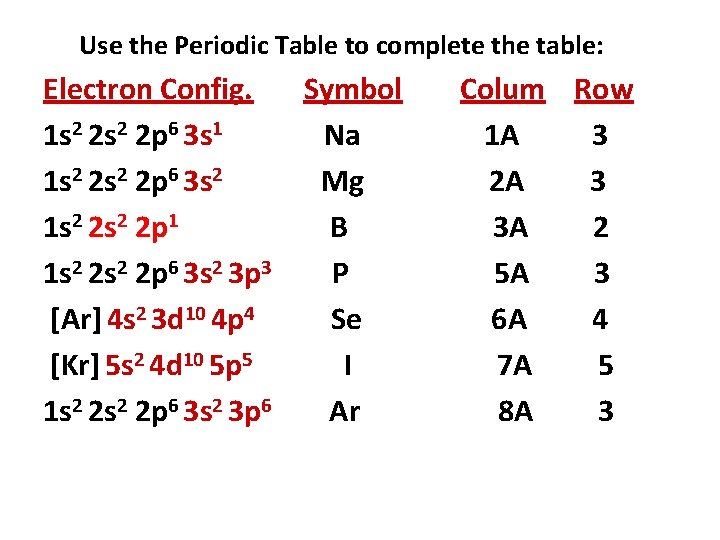
Use the Periodic Table to complete the table: Electron Config. Symbol 1 s 2 2 p 6 3 s 1 Na 1 s 2 2 p 6 3 s 2 1 s 2 2 p 1 1 s 2 2 p 6 3 s 2 3 p 3 [Ar] 4 s 2 3 d 10 4 p 4 [Kr] 5 s 2 4 d 10 5 p 5 1 s 2 2 p 6 3 s 2 3 p 6 Column Row 1 A 3

Use the Periodic Table to complete the table: Electron Config. Symbol 1 s 2 2 p 6 3 s 1 Na 1 s 2 2 p 6 3 s 2 Mg 1 s 2 2 p 1 1 s 2 2 p 6 3 s 2 3 p 3 [Ar] 4 s 2 3 d 10 4 p 4 [Kr] 5 s 2 4 d 10 5 p 5 1 s 2 2 p 6 3 s 2 3 p 6 Colum Row 1 A 3 2 A 3

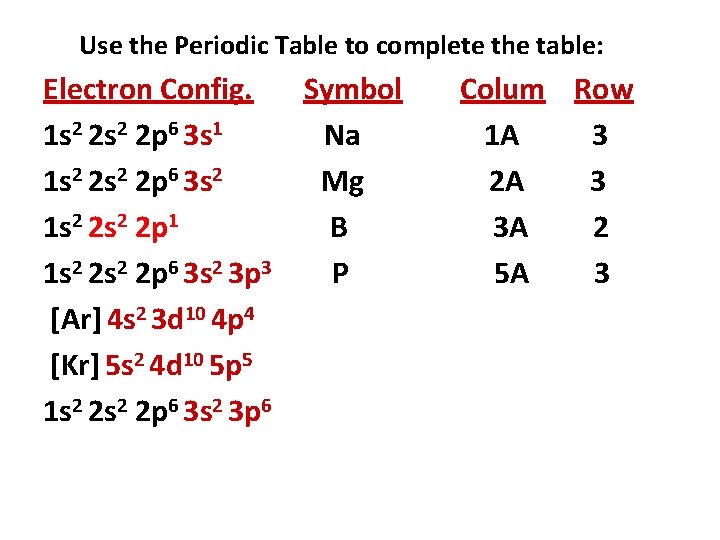
Use the Periodic Table to complete the table: Electron Config. Symbol 1 s 2 2 p 6 3 s 1 Na 1 s 2 2 p 6 3 s 2 Mg 1 s 2 2 p 1 B 1 s 2 2 p 6 3 s 2 3 p 3 [Ar] 4 s 2 3 d 10 4 p 4 [Kr] 5 s 2 4 d 10 5 p 5 1 s 2 2 p 6 3 s 2 3 p 6 Colum Row 1 A 3 2 A 3 3 A 2

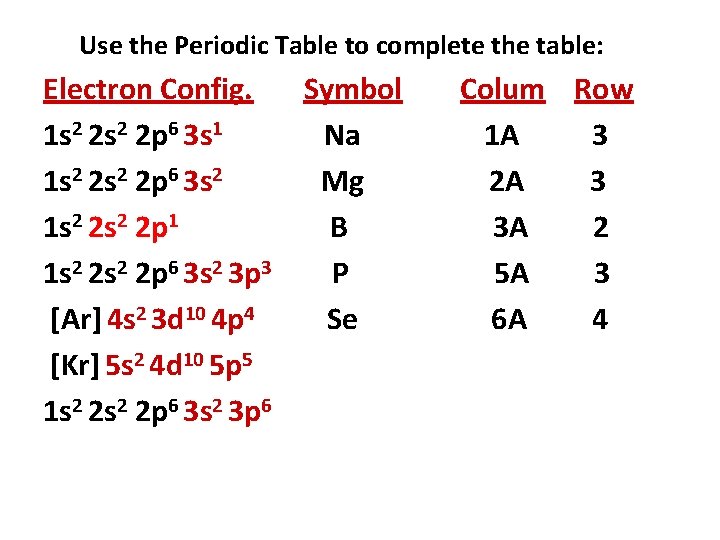
Use the Periodic Table to complete the table: Electron Config. Symbol 1 s 2 2 p 6 3 s 1 Na 1 s 2 2 p 6 3 s 2 Mg 1 s 2 2 p 1 B 1 s 2 2 p 6 3 s 2 3 p 3 P [Ar] 4 s 2 3 d 10 4 p 4 [Kr] 5 s 2 4 d 10 5 p 5 1 s 2 2 p 6 3 s 2 3 p 6 Colum Row 1 A 3 2 A 3 3 A 2 5 A 3

Use the Periodic Table to complete the table: Electron Config. Symbol 1 s 2 2 p 6 3 s 1 Na 1 s 2 2 p 6 3 s 2 Mg 1 s 2 2 p 1 B 1 s 2 2 p 6 3 s 2 3 p 3 P [Ar] 4 s 2 3 d 10 4 p 4 Se [Kr] 5 s 2 4 d 10 5 p 5 1 s 2 2 p 6 3 s 2 3 p 6 Colum Row 1 A 3 2 A 3 3 A 2 5 A 3 6 A 4

Use the Periodic Table to complete the table: Electron Config. Symbol 1 s 2 2 p 6 3 s 1 Na 1 s 2 2 p 6 3 s 2 Mg 1 s 2 2 p 1 B 1 s 2 2 p 6 3 s 2 3 p 3 P [Ar] 4 s 2 3 d 10 4 p 4 Se [Kr] 5 s 2 4 d 10 5 p 5 I 1 s 2 2 p 6 3 s 2 3 p 6 Colum Row 1 A 3 2 A 3 3 A 2 5 A 3 6 A 4 7 A 5

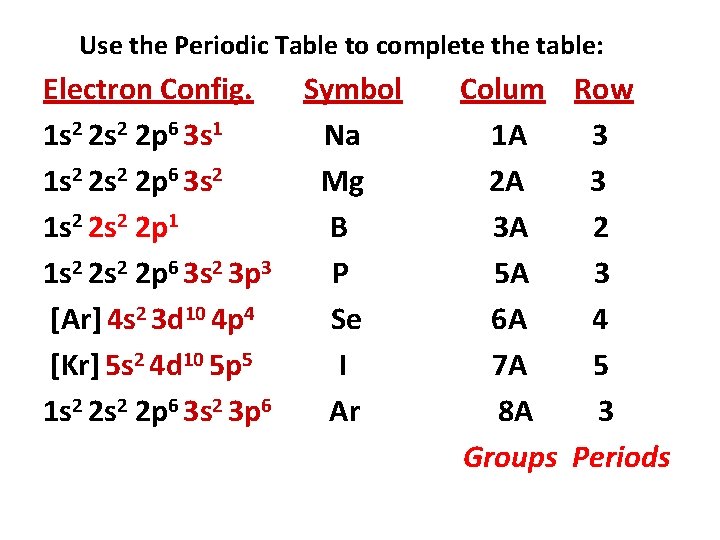
Use the Periodic Table to complete the table: Electron Config. Symbol 1 s 2 2 p 6 3 s 1 Na 1 s 2 2 p 6 3 s 2 Mg 1 s 2 2 p 1 B 1 s 2 2 p 6 3 s 2 3 p 3 P [Ar] 4 s 2 3 d 10 4 p 4 Se [Kr] 5 s 2 4 d 10 5 p 5 I 1 s 2 2 p 6 3 s 2 3 p 6 Ar Colum Row 1 A 3 2 A 3 3 A 2 5 A 3 6 A 4 7 A 5 8 A 3

Use the Periodic Table to complete the table: Electron Config. Symbol 1 s 2 2 p 6 3 s 1 Na 1 s 2 2 p 6 3 s 2 Mg 1 s 2 2 p 1 B 1 s 2 2 p 6 3 s 2 3 p 3 P [Ar] 4 s 2 3 d 10 4 p 4 Se [Kr] 5 s 2 4 d 10 5 p 5 I 1 s 2 2 p 6 3 s 2 3 p 6 Ar Colum Row 1 A 3 2 A 3 3 A 2 5 A 3 6 A 4 7 A 5 8 A 3 Groups Periods

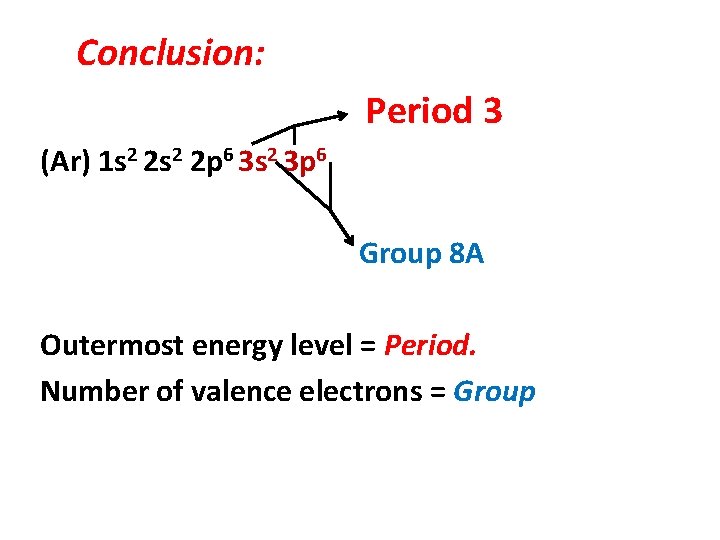
Conclusion: Period 3 (Ar) 1 s 2 2 p 6 3 s 2 3 p 6 Group 8 A Outermost energy level = Period. Number of valence electrons = Group

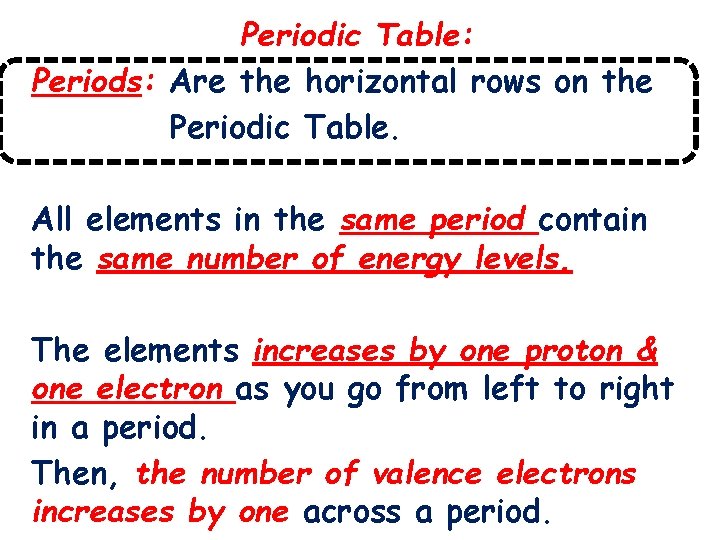
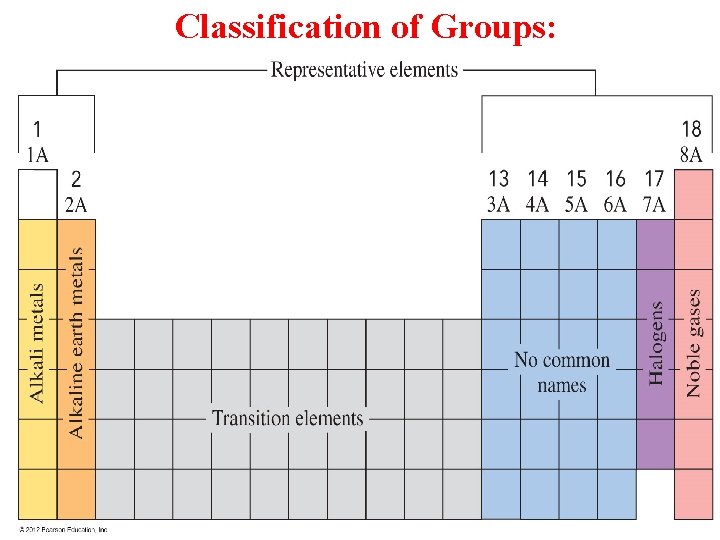
Periodic Table: Periods: Are the horizontal rows on the Periodic Table. All elements in the same period contain the same number of energy levels. The elements increases by one proton & one electron as you go from left to right in a period. Then, the number of valence electrons increases by one across a period.

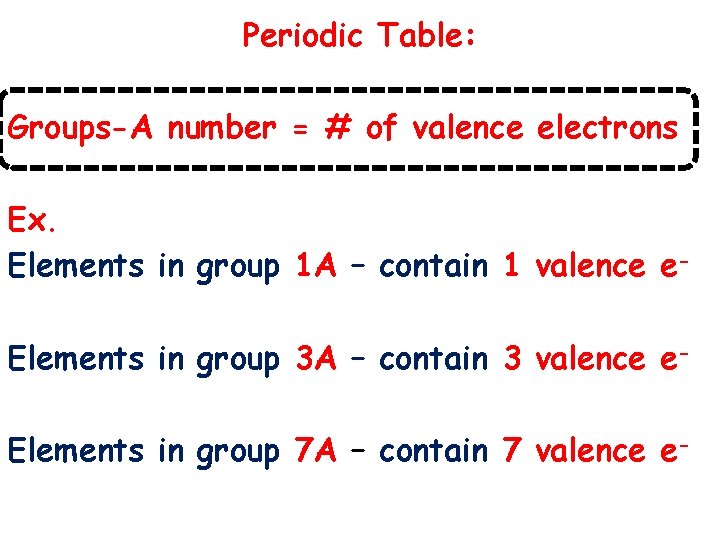
Periodic Table: Groups-A number = # of valence electrons Ex. Elements in group 1 A – contain 1 valence e. Elements in group 3 A – contain 3 valence e. Elements in group 7 A – contain 7 valence e-

Periodic Table: Groups: Vertical columns (elements in the same group have similar properties). Group 1 A: The most reactive metals (Alkali metals). Group 2 A : Reactive metals also (Alkaline Earth metals). Group 7 A: The most reactive non metals (Halogens). Group 8 A: Unreactive, very stables.

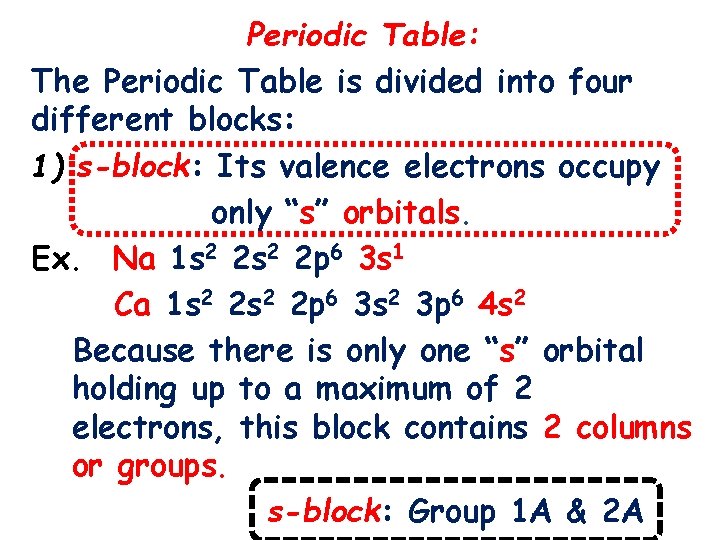
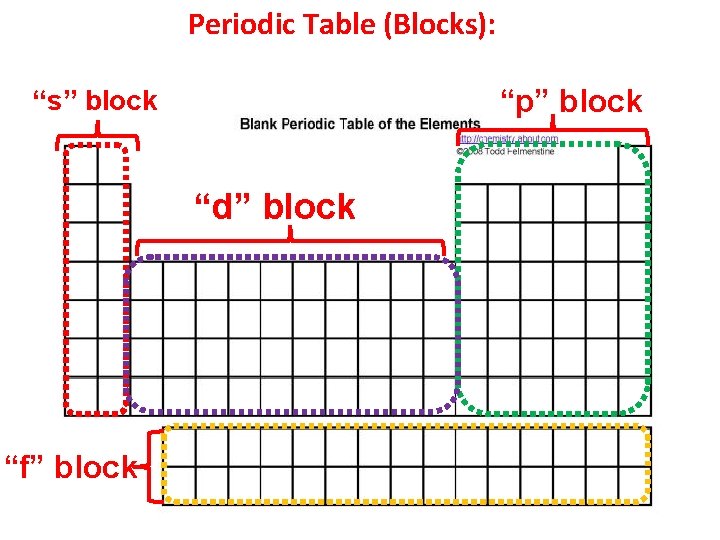
Periodic Table: The Periodic Table is divided into four different blocks: 1) s-block: Its valence electrons occupy only “s” orbitals. Ex. Na 1 s 2 2 p 6 3 s 1 Ca 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 Because there is only one “s” orbital holding up to a maximum of 2 electrons, this block contains 2 columns or groups. s-block: Group 1 A & 2 A

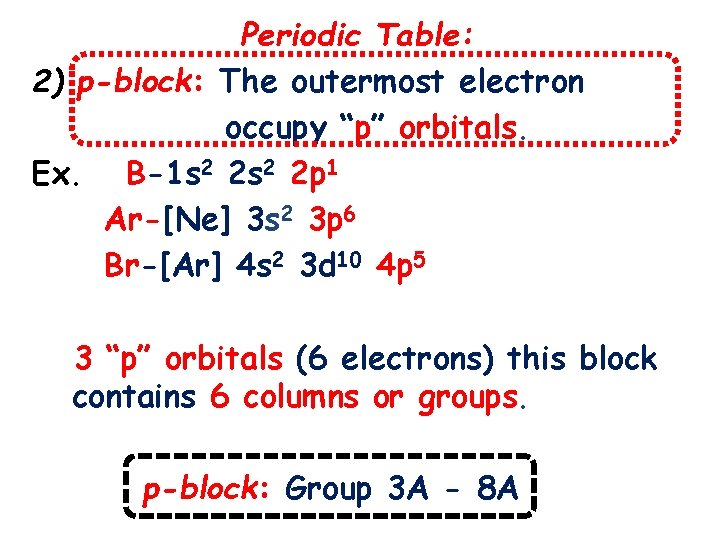
Periodic Table: 2) p-block: The outermost electron occupy “p” orbitals. Ex. B-1 s 2 2 p 1 Ar-[Ne] 3 s 2 3 p 6 Br-[Ar] 4 s 2 3 d 10 4 p 5 3 “p” orbitals (6 electrons) this block contains 6 columns or groups. p-block: Group 3 A - 8 A

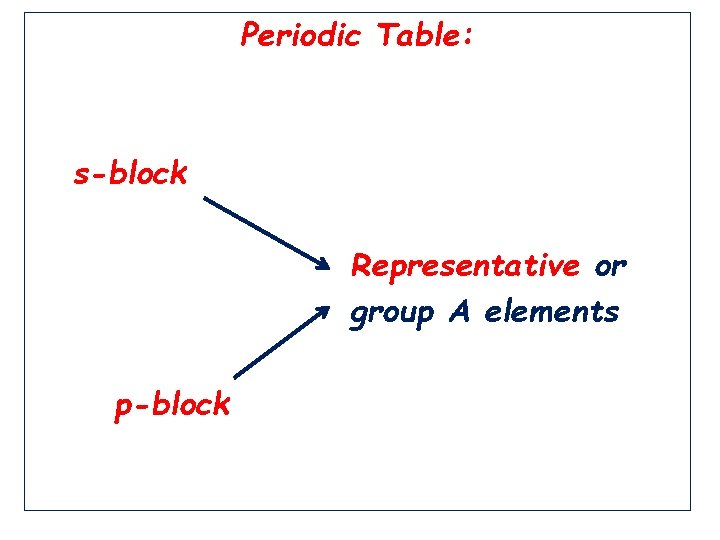
Periodic Table: s-block Representative or group A elements p-block

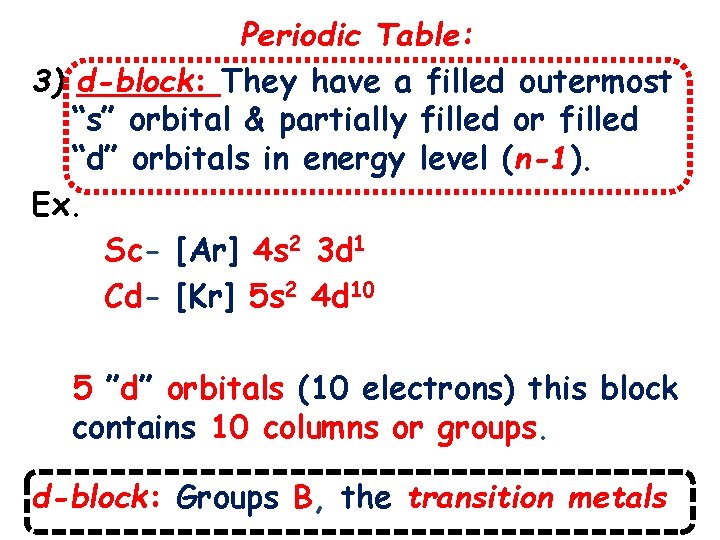
Periodic Table: 3) d-block: They have a filled outermost “s” orbital & partially filled or filled “d” orbitals in energy level (n-1). Ex. Sc- [Ar] 4 s 2 3 d 1 Cd- [Kr] 5 s 2 4 d 10 5 ”d” orbitals (10 electrons) this block contains 10 columns or groups. d-block: Groups B, the transition metals
![Periodic Table 4 fblock The outermost electron is placed in f orbitals Ex CeXe Periodic Table: 4) f-block: The outermost electron is placed in “f” orbitals. Ex. Ce-[Xe]](https://slidetodoc.com/presentation_image_h2/52ff620e29f05368c7ce5a7bf2656f08/image-23.jpg)
Periodic Table: 4) f-block: The outermost electron is placed in “f” orbitals. Ex. Ce-[Xe] 6 s 2 4 f 2 Np- [Rn] 7 s 2 5 f 5 7 “f” orbitals (14 electrons) this block contains 14 columns or groups. f-block: The inner transition metals

Periodic Table (Blocks): “p” block “s” block “d” block “f” block

Classification of Groups: 25
 Metals vs nonmetals vs metalloids
Metals vs nonmetals vs metalloids Metals on periodic table
Metals on periodic table Metals nonmetals semimetals
Metals nonmetals semimetals Compare metals nonmetals and metalloids
Compare metals nonmetals and metalloids Metals nonmetals and metalloids answer key
Metals nonmetals and metalloids answer key Compare metals nonmetals and metalloids
Compare metals nonmetals and metalloids Metals nonmetals and metalloids difference
Metals nonmetals and metalloids difference Non metals that conduct electricity
Non metals that conduct electricity Properties of metals nonmetals and semimetals
Properties of metals nonmetals and semimetals Brittle nonmetals
Brittle nonmetals Periodic table divided in metals nonmetals and metalloids
Periodic table divided in metals nonmetals and metalloids Poem about metals nonmetals and metalloids
Poem about metals nonmetals and metalloids Periodic table metals nonmetals metalloids noble gases
Periodic table metals nonmetals metalloids noble gases Periodic table metals nonmetals metalloids
Periodic table metals nonmetals metalloids Periodic metals and nonmetals
Periodic metals and nonmetals Least reactive non-metal
Least reactive non-metal Difference between metal oxides and non metal oxides
Difference between metal oxides and non metal oxides Uses for non metals
Uses for non metals Optical properties of metals and nonmetals
Optical properties of metals and nonmetals Chapter 4 metals and nonmetals
Chapter 4 metals and nonmetals Nonmetals on the periodic table
Nonmetals on the periodic table Metals vs nonmetals properties
Metals vs nonmetals properties Metal and non metal example
Metal and non metal example Non metals
Non metals Characteristics of metalloids
Characteristics of metalloids Periodic table of elements regents
Periodic table of elements regents