INTRODUCTION TO METALS NONMETALS AND METALLOIDS Groups of


- Slides: 8

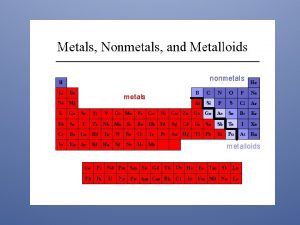
INTRODUCTION TO METALS, NONMETALS AND METALLOIDS

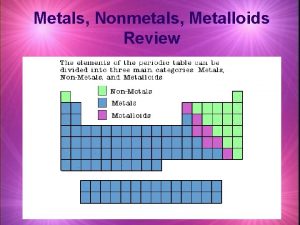
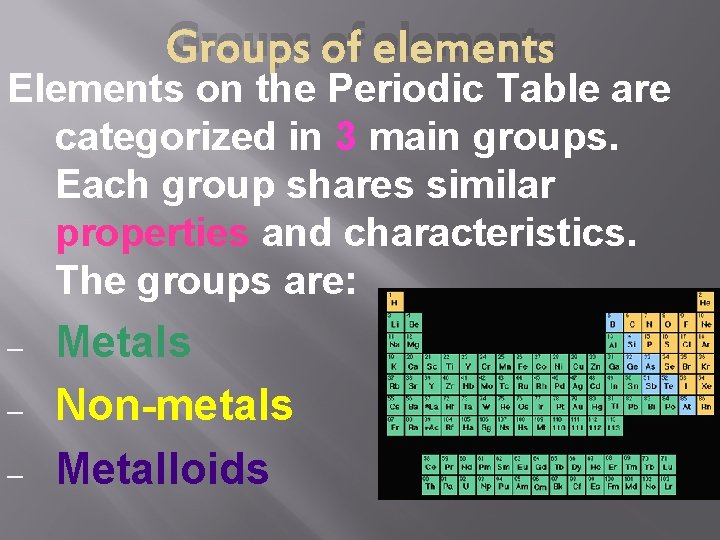
Groups of elements Elements on the Periodic Table are categorized in 3 main groups. Each group shares similar properties and characteristics. The groups are: – – – Metals Non-metals Metalloids

Metals make up almost 75% of the Periodic Table Found on left side and middle of the Periodic Table (excluding hydrogen). Characteristics: Have luster (Shiny) Good conductor of heat and electricity: Silver (Ag) and copper (Cu) are some of the most efficient metals and are often used in electronics. Solids (except Mercury) Malleable – able to pound into a shape (flexible) Ductile – able to draw into wires.

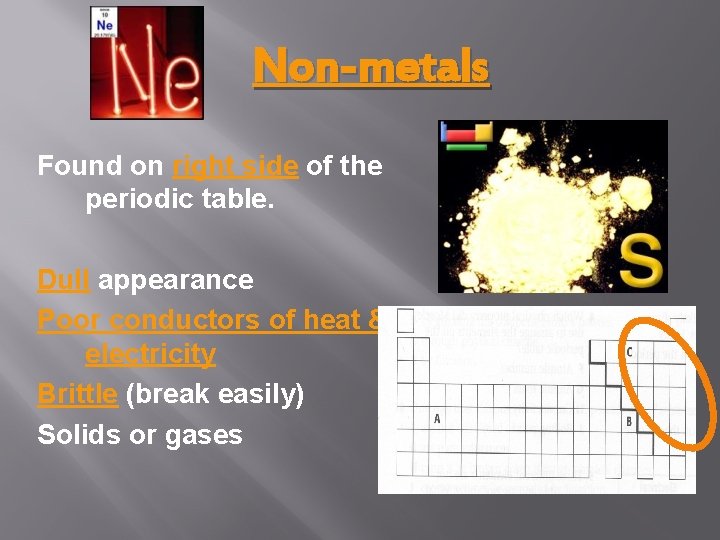
Non-metals Found on right side of the periodic table. Dull appearance Poor conductors of heat & electricity Brittle (break easily) Solids or gases

Metalloids Found between metals and non-metals in a stair step line. Elements that have characteristics of both metals and nonmetals. Specific characteristics depend on the element. All solids, can be shiny or dull, they are conductors but not as good as metals (semi-conductors). Boron Silicon Germanium Arsenic Antimony Tellurium Polonium Astatine

Noble Gases The far right column (group 18) on the periodic table. Sometimes considered to be non-metals, but often an independent group because they do NOT react with other elements Very stable and non-reactive elements. All gases

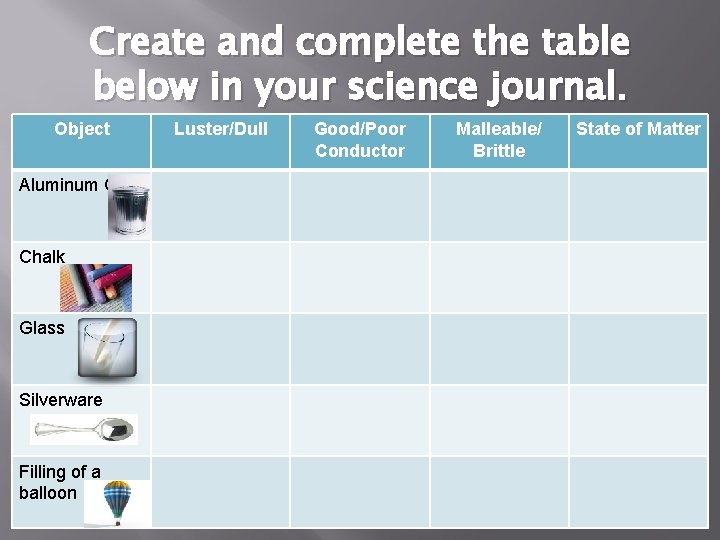
Create and complete the table below in your science journal. Object Aluminum Can Chalk Glass Silverware Filling of a balloon Luster/Dull Good/Poor Conductor Malleable/ Brittle State of Matter

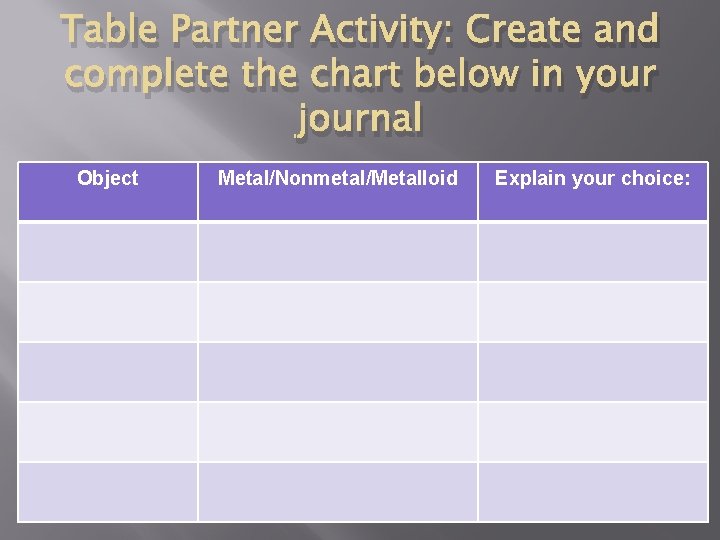
Table Partner Activity: Create and complete the chart below in your journal Object Metal/Nonmetal/Metalloid Explain your choice:
 Metals vs nonmetals
Metals vs nonmetals Periodic table metals and nonmetals
Periodic table metals and nonmetals I am malleable, but i do not have a shiny luster.
I am malleable, but i do not have a shiny luster. Periodic table metals nonmetals metalloids
Periodic table metals nonmetals metalloids Metals nonmetals and metalloids answer key
Metals nonmetals and metalloids answer key Metals vs nonmetals vs metalloids
Metals vs nonmetals vs metalloids Metals nonmetals and metalloids difference
Metals nonmetals and metalloids difference Non metals that can conduct electricity
Non metals that can conduct electricity Metals nonmetals and metalloids chart
Metals nonmetals and metalloids chart