Physical Properties of Metals NonMetals and Metalloids Physical








- Slides: 11

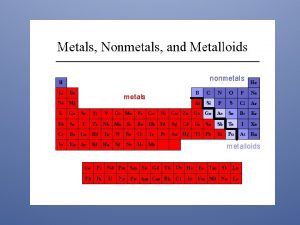
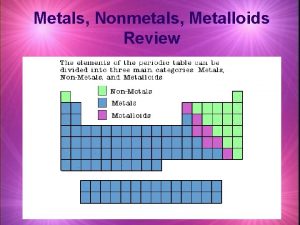
Physical Properties of Metals, Non-Metals, and Metalloids

Physical Properties • Definition: Properties that can be observed without making any chemical changes to the substance. • Determined by the atomic structure of the substance and the strength of the bonds.

Examples that could be easily observed in your classrooms: – Color – not always diagnostic, but sometimes can be – Luster – how the light reflects off the surface of a substance. – Malleability – the ability of a substance to be deformed under compressive stress. – Density – mass/unit volume. – Conductivity – the ability of the substance to transmit electricity or heat.

Color

Luster Metallic Non-Metallic

Malleability

Density

Conductivity

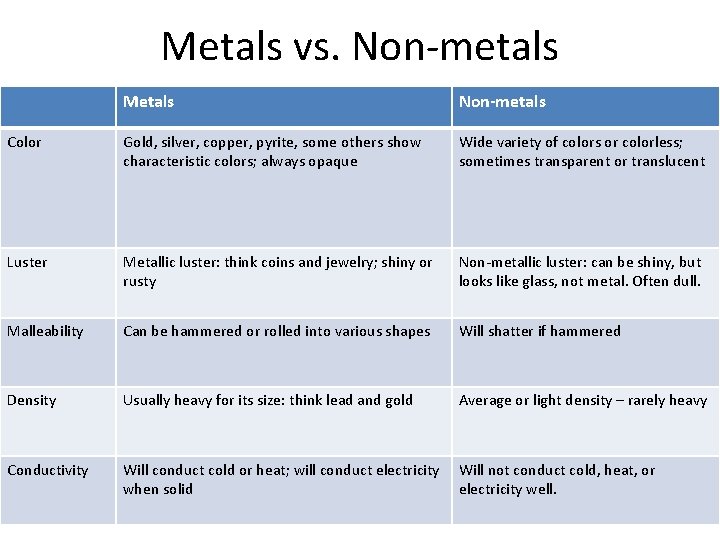
Metals vs. Non-metals Metals Non-metals Color Gold, silver, copper, pyrite, some others show characteristic colors; always opaque Wide variety of colors or colorless; sometimes transparent or translucent Luster Metallic luster: think coins and jewelry; shiny or rusty Non-metallic luster: can be shiny, but looks like glass, not metal. Often dull. Malleability Can be hammered or rolled into various shapes Will shatter if hammered Density Usually heavy for its size: think lead and gold Average or light density – rarely heavy Conductivity Will conduct cold or heat; will conduct electricity when solid Will not conduct cold, heat, or electricity well.

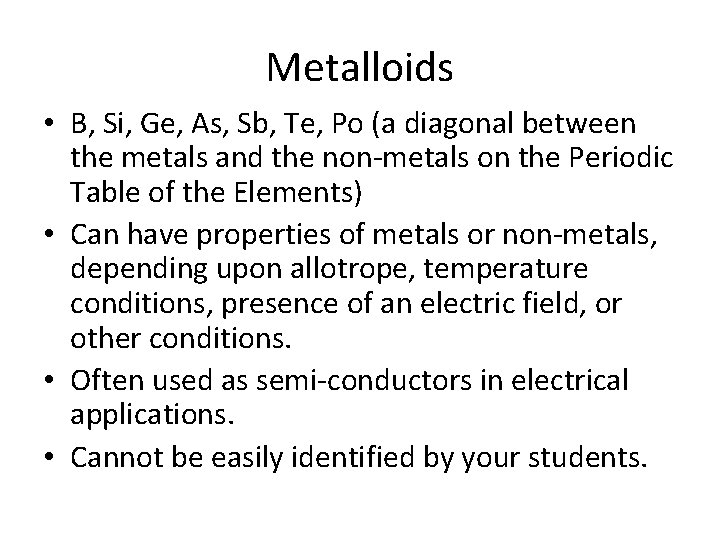
Metalloids • B, Si, Ge, As, Sb, Te, Po (a diagonal between the metals and the non-metals on the Periodic Table of the Elements) • Can have properties of metals or non-metals, depending upon allotrope, temperature conditions, presence of an electric field, or other conditions. • Often used as semi-conductors in electrical applications. • Cannot be easily identified by your students.

For More Information, IRC: Physical Properties of Metals, Non-metals, and Metalloids
 Characteristics of metals
Characteristics of metals Non metals in the periodic table
Non metals in the periodic table Periodic table metals nonmetals and metalloids
Periodic table metals nonmetals and metalloids Bulletproof metal periodic table
Bulletproof metal periodic table Non metals
Non metals Metals vs nonmetals vs metalloids
Metals vs nonmetals vs metalloids Metals nonmetals and metalloids difference
Metals nonmetals and metalloids difference Metals nonmetals and metalloids periodic table
Metals nonmetals and metalloids periodic table Is chalk a metal or nonmetal
Is chalk a metal or nonmetal Metals vs nonmetals vs metalloids
Metals vs nonmetals vs metalloids Periodic table divided in metals nonmetals and metalloids
Periodic table divided in metals nonmetals and metalloids Poem about metals nonmetals and metalloids
Poem about metals nonmetals and metalloids