Metals Nonmetals and Metalloids Metals l l l






- Slides: 7

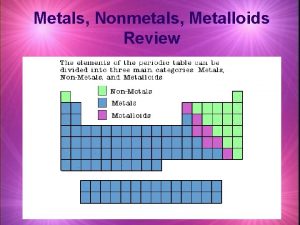
Metals, Nonmetals and Metalloids

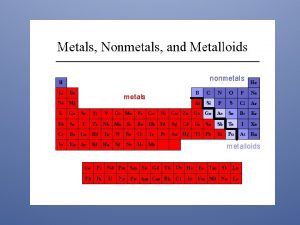
Metals l l l Location: 2/3 of all elements, left of the ladder, most active lower left (Fr) Lose/gain: lose electrons to form positive ions (cations) Ionization energy (I. E. energy absorbed when electrons are lost from a neutral atom in the gaseous phase-table S)Low Electronegativity (E. N. tendency for an atom to attract electrons to itself when it chemically combines with other elements-table S)Low Electron Affinity (E. A. energy gained when electrons are gained by a neutral atom in the gaseous phase)Low

Metals l Other Properties: Luster, Malleability, Ductility, High thermal and electrical conductivity. l Physical State and examples: Solids except mercury (liquid) at room temperature.

Nonmetals l Location: 1/3 of elements, right of ladder, most active upper right (F) l Lose/gain: gain electrons to form negative ions (anions) l I. E. : high l E. N. : high l E. A. : high

Nonmetals l Other properties: Dull, brittle, not ductile, not malleable, poor thermal and electrical conductors l Physical state and examples: Gases (oxygen, nitrogen), molecular solids or network solids at room temperature (carbon ) or a liquid (only bromine)

Metalloids (semi-metals) l Location: along the ladder, 7 of them (B, Si, Ge, As, Sb, Te, and At) (note: Al and Po are metals) l Lose/gain: lose, gain or share electrons depending upon what it bonds to. Can form either positive or negative ions. l I. E. : medium l E. N. : medium l E. A. : medium

Metalloids l Other Properties: Have some properties of metals and others of nonmetals. Generally intermediate in luster and conductivity. l Physical state and examples: most are solids (B, Sb, Te etc. )