Metals NonMetals Metalloids Chapter 5 Sections 3 and














- Slides: 16

Metals, Non-Metals, Metalloids Chapter 5 Sections 3 and 4

Metals are elements that are good conductors of electric current and heat. Metals tend to be shiny and bendable- like copper wire. Most of the elements in the periodic table are. metals

Physical Properties of Metals Physical properties of metals include luster, malleability, ductility, and conductivity.


Luster is shiny and reflective.

Malleable Material that can be hammered or rolled into flat sheets or other shapes.

Ductile Material is one that can be pulled out or drawn, into long wires.

Thermal Conductivity Ability of an object to transfer heat

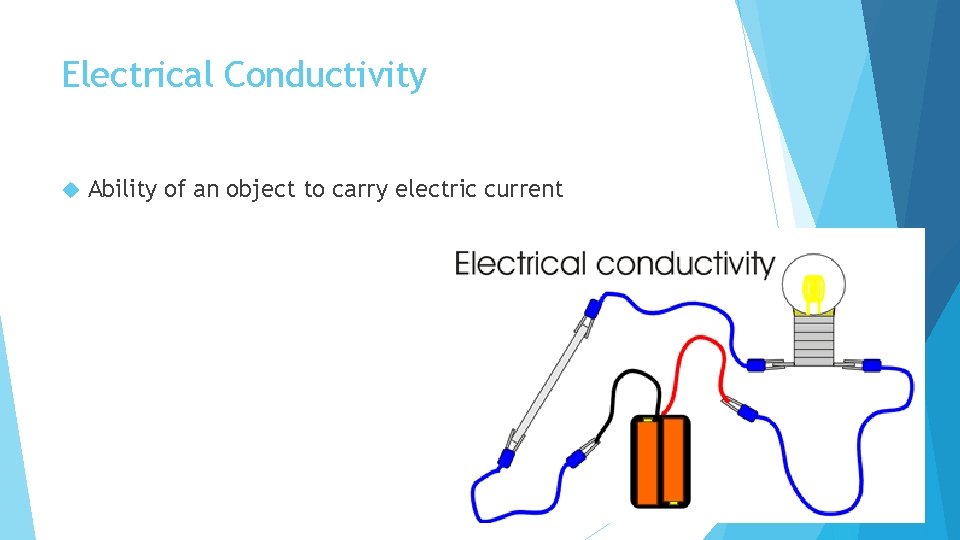
Electrical Conductivity Ability of an object to carry electric current

Chemical Properties Reactivity- ease and speed with which an element combines or reacts with other substances. Sodium (Na) reacts strongly with water. Gold (Au) do not react easily with other substances. Iron reacts slowly with oxygen in the air forming iron oxide, or rust. The deterioration of a metal due to a chemical reaction in the environment is called corrosion.


Alkali Metals or Group 1 These metals are the most reactive metals in the periodic table.

Alkaline Earth Metals- Group 2 These metals are harder and denser, and melt at higher temperatures than alkali metals. These metals are very reactive; however, not as reactive as alkali metals of group 1.

Transition Metals- Group 3 -12 Transition metals include iron, copper, nickel, gold, and silver. Most of these metals are hard and shiny solids. Mercury is a liquid at room temperature. These metals are less reactive than alkali and alkaline metals.

Nonmetals are elements that lack most of the properties of a metal. In general, most nonmetals are poor conductors of electric current and heat. Solid nonmetals tend to be dull and brittle. Hydrogen, Carbon, Nitrogen, Oxygen, and Phosphorus are all nonmetals

Metalloids have some properties of metals and some properties of nonmetals. All metalloids are solids at room temperature. Metalloids are brittle, hard, and somewhat reactive. Metalloids' most useful property is the ability to conduct electric current. Semiconductors- substances that can conduct electric currents under some conditions but not under other conductions.