Metals Vs NonMetals Vs Metalloids Aim How can






- Slides: 12

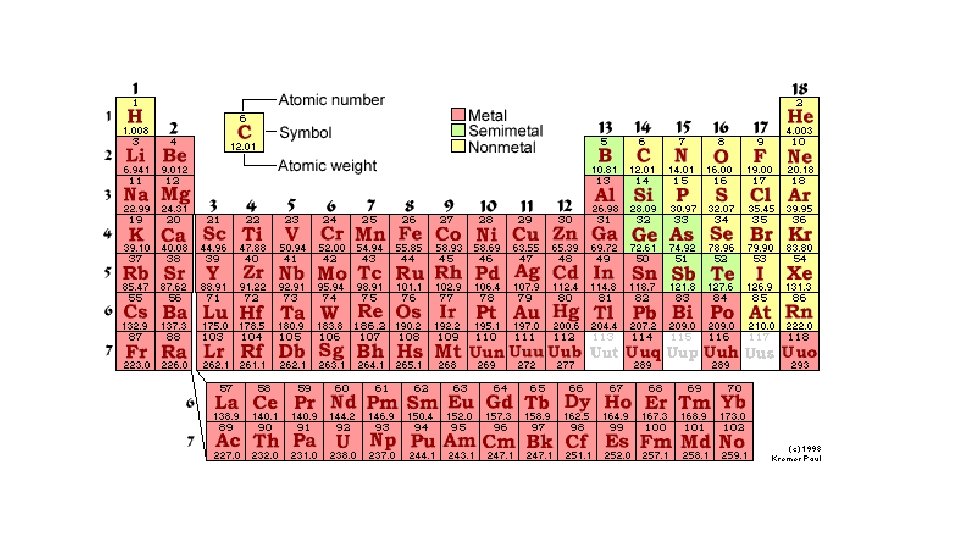
Metals Vs. Non-Metals Vs. Metalloids Aim: How can we further classify and characterize each element on the periodic table?


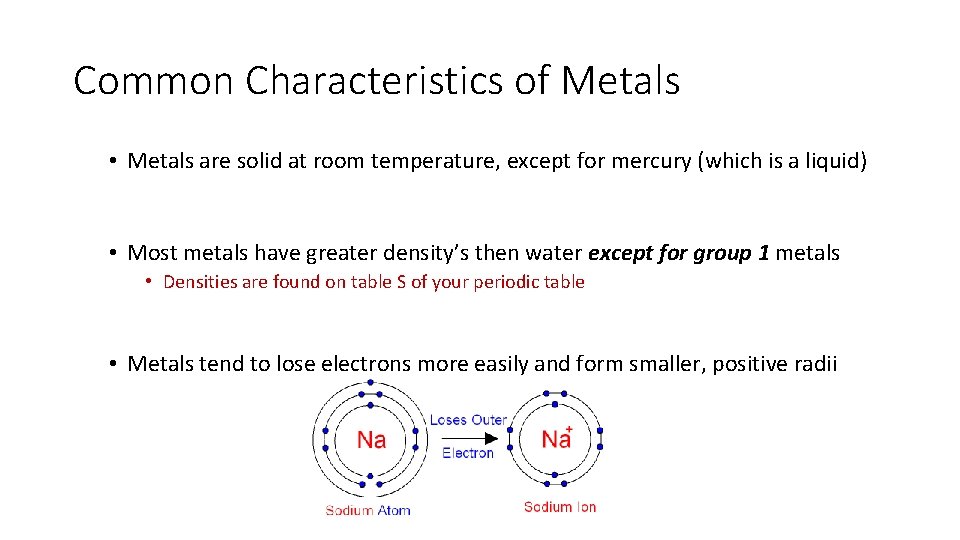
Common Characteristics of Metals • Metals are solid at room temperature, except for mercury (which is a liquid) • Most metals have greater density’s then water except for group 1 metals • Densities are found on table S of your periodic table • Metals tend to lose electrons more easily and form smaller, positive radii

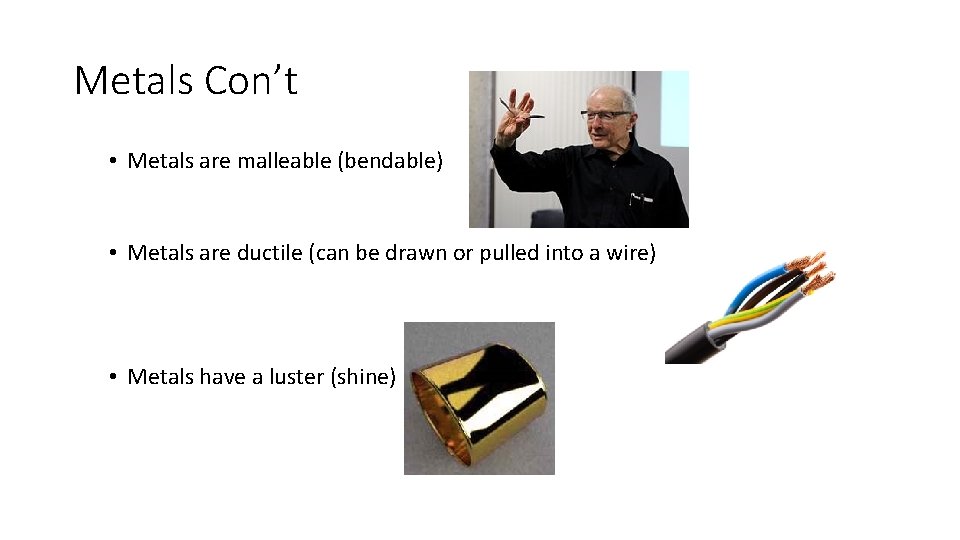
Metals Con’t • Metals are malleable (bendable) • Metals are ductile (can be drawn or pulled into a wire) • Metals have a luster (shine)

Metals con’t • Metals are good conductors of heat and electricity • This means that electrons/charged particles are moving around • Let’s see how this works Electron conductivity Tester

Metals: Electronegativity and Ionization Energy • Metals have low ionization energies and electronegativities • Ionization energy is the amount of energy needed to remove a valance electron. Because metals lose electrons, it does not take much energy to remove a valance electron. • Electronegativity is the level of attraction of a nucleus for a bonded electron. Because metals are losing electrons, they have a low electronegativity

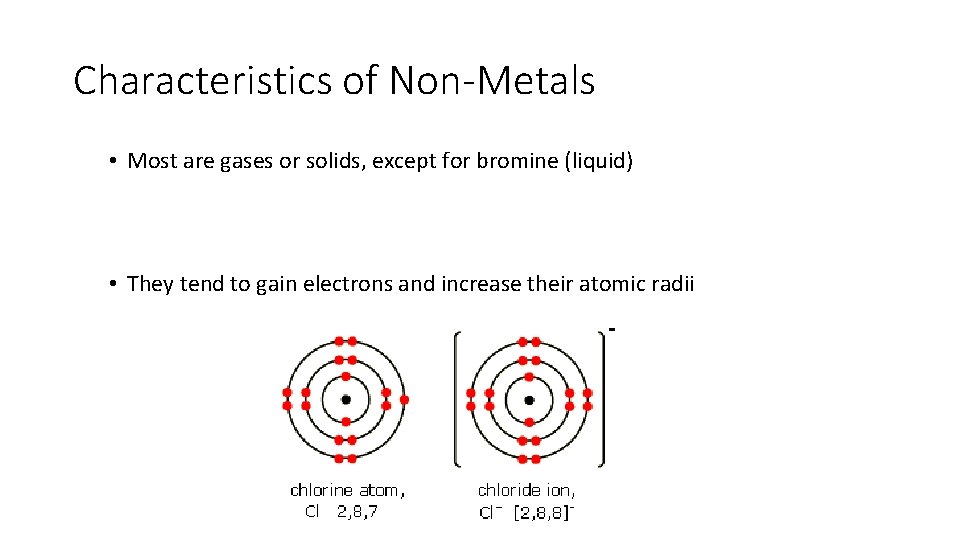
Characteristics of Non-Metals • Most are gases or solids, except for bromine (liquid) • They tend to gain electrons and increase their atomic radii

Non-Metals Con’t • They are not malleable (bendable) • They tend to be brittle in the solid phase • Solid non-metals lack luster; their surface is dull

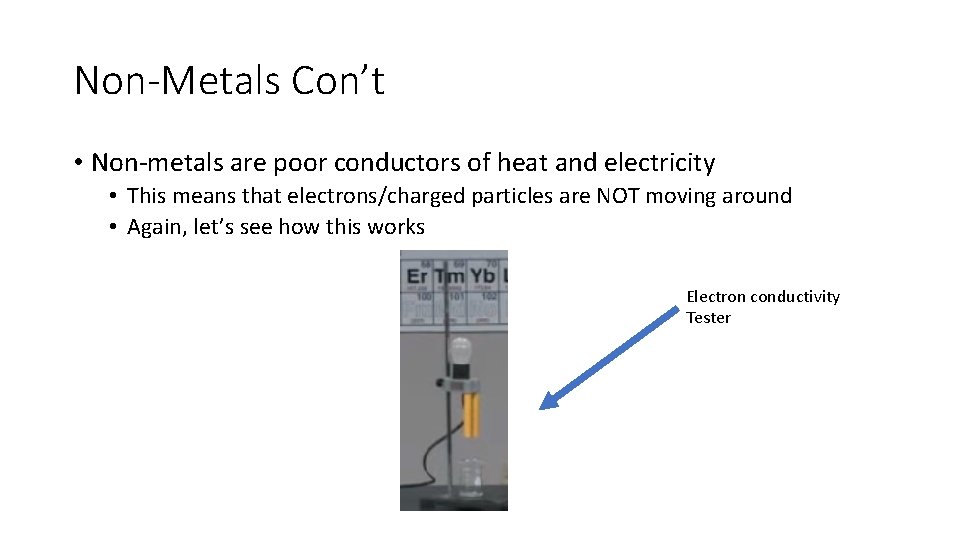
Non-Metals Con’t • Non-metals are poor conductors of heat and electricity • This means that electrons/charged particles are NOT moving around • Again, let’s see how this works Electron conductivity Tester

Non-Metals: Electronegativity and Ionization Energy • They have high electronegativity and ionization energies • Ionization energy is the amount of energy needed to remove a valance electron. Because non-metals gain electrons, it takes a lot of energy to remove a valance electron. • Electronegativity is the level of attraction of a nucleus for a bonded electron. Because non-metals gain electrons, they have a high electronegativity

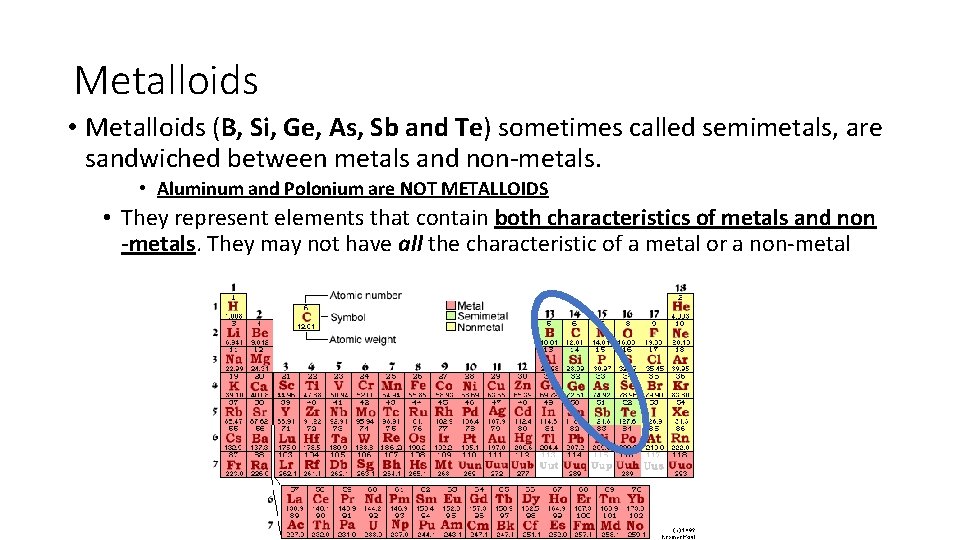
Metalloids • Metalloids (B, Si, Ge, As, Sb and Te) sometimes called semimetals, are sandwiched between metals and non-metals. • Aluminum and Polonium are NOT METALLOIDS • They represent elements that contain both characteristics of metals and non -metals. They may not have all the characteristic of a metal or a non-metal

Some Questions: • Each lab table/ row will get four items. It is your job to determine whether these items are metals or non-metals. Use the notes we took to support your reasoning. • Once you finish, look through your things. Pick one metal and one non-metal. Use the notes we took to support your reasoning.